
Pragmatic management of myogenous temporomandibular disorder—a narrative review
Introduction
Background
Masticatory myofascial pain, the most common type of temporomandibular disorder (TMD) refers to chronic pain that originates from the jaw muscles/myofascial and associated soft tissues. It affects nearly 5 million individuals in the US (1-3). The International Classification of Orofacial Pain (ICOP) subcategorizes myofascial pain into that of primary and secondary origin.
Rationale and knowledge gap
Myofascial pain secondary to acute soft tissue injury and inflammation is usually easily diagnosed and managed, with the resolution of pain concomitant with tissue healing. However, understanding primary myofascial pain (referred to as ‘mTMD’ in this manuscript) and the cause of pain persistence after tissue healing have been the significant challenges (4-7).
Objective
The key questions addressed in this narrative review are twofold—what forms the basis for our current understanding of masticatory myogenous TMD’s etiology and diagnosis and how our understanding can impact its management. We present the following article in accordance with the Narrative Review reporting checklist (available at https://joma.amegroups.com/article/view/10.21037/joma-22-14/rc).
Methods
The method employed for the narrative review is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1/15/2022–3/15/2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Temporomandibular disorder |
| Timeframe | Contemporary PubMed literature up to March 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: Review, Research publications, case reports and series in English language |
| Selection process | By author opinion and collaborative discussion |
Discussion
Current understanding and basis of management of myofascial TMD (mTMD)
To date, the diagnosis of mTMD relies largely on patient history and clinical examination (8). In the absence of definite histopathology and of any quantitative assessment to corroborate clinical impression, the practitioner’s clinical assessment is subjective and variable. mTMD is often characterized by the presence of myofascial trigger points, described as tender areas within taut bands of skeletal muscles, that when stimulated by palpation, produces the pain that spreads to the surrounding area, or refers to distant sites (4,9). Clinically, trigger points are subdivided into active and latent. Both are painful upon palpation, but only the former reproduces the patient’s chief pain complaint (5,9).
The definition and etiology of myofascial pain are not fully understood. For instance, the role of the pathognomonic trigger points in mTMD pain has generated much fundamental debate, including whether they are the result or the cause of myofascial pain, even whether they are necessary or sufficient for developing myofascial pain is debatable. In fact, the 2020 National Institutes of Health (NIH) Helping to End Addiction Long-term Initiative Workshop on Myofascial Pain acknowledged this critical question’s challenge and scientific opportunity (10). Many theories have been proposed to explain myofascial pain without rigorous validation.
Some hypotheses of primary myofascial pain development suggest that abnormal muscle load, such as sustained contraction or repetitive movements, or malfunction of the muscle motor end-plate with spontaneous release and increased availability of acetylcholine, cause peripheral inflammation and potential tissue damage (11-15). This may additionally result in peripheral muscle nociceptor and central sensitization.
Fascial tissues investing all muscle tissues are in close contact with the muscle fibers, and its generous innervation may likely contribute to myofascial pain pathophysiology (10). The Myofascial unit hypothesis implicates the fascial tissues as a significant contributing factor in myofascial pain pathogenesis. The fascial tissues house the muscle spindles and Golgi corpuscles and contain free nerve endings capable of transmitting pain in certain conditions (16). The fascial tissues demonstrate changes with age and exhibit responses to sex hormones and endocannabinoids, consistent with mTMD risk factors such as increasing age, female sex, and also emotional/psychological burden (3,17). Clinical manifestations of peripheral sensitization would comprise complaints of pain with jaw function or increased pain due to local pressure (11,12,15).
The increased and persistent nociceptive input from the central nervous system from the sensitized peripheral afferents may result in secondary afferent hyperexcitability, a phenomenon also known as central sensitization, and can explain painful pain features observed in mTMD, such as spontaneous pain and pain extending beyond the original site of injury (18,19).
It is conceivable that all these theories hold true and that multiple mechanisms/combinations of mechanisms may be operational in the pathogenesis of myofascial pain. Thus, the etiology of masticatory myofascial pain may be multifactorial. This can explain the heterogeneous and complex clinical presentation of mTMD. mTMD can present predominantly as a ‘local’ phenomenon, confined to the masticatory myofascial tissue or as more ‘global’ in its extent involving mechanisms at the central nervous system level. These phenotypes were observed in the OPPERA (Orofacial Pain: Prospective Evaluation and Risk Assessment) study (3,17).
mTMDs in the kaleidoscope of chronic pain
The OPPERA study was a seminal, large population-based prospective study designed to identify the risk factors that contribute to the onset and persistence of TMDs. These risk factors were identified as biopsychosocial, environmental or genetic (20). Some of the self-reported factors in subjects with painful TMDs were greater occurrence of trauma to the jaw, parafunctional behaviors, increased pain intensity in the face and jaw area, modification of pain by jaw function, stiffness or cramping, joint noises, headaches, and even, chronic pain in other parts of the body (3,17).
It was notable that the prevalence of high-impact pain, defined as high-intensity pain or moderate/high levels of self-reported pain-related interference, was nearly four times higher among those with orofacial pain than without. Further, individuals with high impact pain had higher pain sensitization and more significant tenderness to palpation of multiple body sites (3,17,21).
The psychosocial risk factors for chronic TMD subjects included higher levels of psychological and affective distress, greater stress perception and catastrophizing, and increased somatic awareness (22).
The OPPERA study clustered individuals who had a higher risk of developing painful TMDs- namely, the adaptive, pain-sensitive, and global symptoms clusters. Individuals in the adaptive cluster may have more localized pathology, whereas the individuals in the other two clusters are thought to have more pain sensitivity due to central sensitization (23).
The data suggested that mTMD could either be an isolated presentation or a part of a constellation of phenomena with more ‘generalized’ or ‘central’ mechanisms. Accordingly, TMD pain is often comorbid with migraine, fibromyalgia, and other types of generalized pain.
Management of mTMDs
Perhaps not surprisingly, multiple modalities exist for the management of myofascial pain- dry needling, trigger point injections, oral appliances, acupuncture, and tissue mobilization, to name a few (24,25). A recent meta-analysis of a systematic review on treatments for myogenous TMD suggested that the most efficacious therapies were manual therapy, counseling, local anesthesia (trigger point injections) and appliance therapy; however, there is a considerable level of controversy, mainly due to methodological heterogeneity. This results in a low level of evidence for most treatments and underscores the need for better quality studies (26,27).
Counseling and self-care
Counseling may vary from patient education regarding the condition, prognosis, and self-care techniques, to more specific cognitive-behavioral therapy (CBT). Counseling is usually combined with other treatment modalities and seems beneficial for both acute and chronic conditions (28).
Intraoral appliance
The oral appliance is the most common therapeutic intervention for mTMD (27). Multiple designs of oral appliances are described in the literature. A full-coverage hard acrylic appliance, covering either the maxillary or mandibular arch, with bilateral centric contacts against opposing teeth, especially for long-term use, may be recommended to reduce the chances of occlusal changes. Cost, time-lapse for fabrication, and regular maintenance might be some of its disadvantages but most importantly are the caution towards the proper fabrication and patient’s instruction regarding its use (25,29).
Physiotherapy
Tissue manipulation, also known as manual manipulation or therapy, includes manipulation of soft tissues and joints of the head and neck. There are different techniques, and the generally desired outcomes include improvement of muscle spasm, local circulation, and adhesions with increased range of motion and pain. This therapy requires multiple sessions performed by a physical therapist (30,31).
Trigger point injection
It consists of administering local anesthetic without a vasoconstrictor into an identifiable trigger point in taut bands of skeletal muscles (13). Network meta-analysis of systematic reviews have concluded that local anesthetics delivered as trigger point injections alleviate pain and improve maximum mouth opening for at least 6 months (32). Trigger point injections are technique-sensitive and require the accurate diagnosis, localization, manipulation of the trigger points, and medication delivery (33).
Dry needling
Dry needling is a therapeutic modality for myofascial pain usually performed by physical therapists and consists of the insertion of thin solid needles into myofascial trigger points, tendons, ligaments, and scar tissues (34). This modality has been suggested to reduce peripheral pain and sensitization (29).
Acupuncture
Acupuncture is a therapy modality based on Chinese Medicine and consists of inserting multiple thin solid needles in specific points, called acupoints. It is performed by a trained professional in a series of regular visits. Multiple acupuncture methods have been suggested to improve pain-limited mouth opening and quality of life (35). Acupuncture is considered an adjunct to formal therapies for mTMD, due to the limited evidence on its efficacy in mTMD (24).
Additionally, there is emerging support for oral pharmacotherapy with medications such as pregabalin (36).
It is crucial to recognize that the knowledge gap that currently exists in the field in validating the relative effectiveness of various treatment modalities is not one that can be bridged by more critical assessment of existing literature in the form of additional systematic reviews or meta-analyses beyond what already exists in the literature. As can be inferred from this manuscript, multiple such analyses have been performed and published- in contrast, there is a dearth of rigorously designed, prospective comparisons of promising treatment modalities through randomized, multi-blinded (operator-, evaluator- and subject-blinded) clinical trials capable of objective patient assessment, unbiased treatment assignment and outcome measurement. Until such a time, there may be benefit in exploring alternate strategies that lend themselves to simple treatment delivery and less ambiguous assessment of patient response. Recently, we developed an additional modality of treatment- the Temporo-masseteric Nerve block (TMNB), a.k.a. the Twin block, as it was formerly referred to (37-41).
The TMNB as a novel tool in the management of mTMDs
The TMNB is a local anesthetic injection that targets the deep temporal and masseteric branches of the Mandibular division (V3) of the Trigeminal Nerve, the fifth cranial nerve (Figure 1) (40,41). The original impetus behind the development of the TMNB was the argument that interrupting the relay of pain signals from the muscle would relieve the patient’s pain symptoms, regardless of whether the origin of pain were trigger points in the muscle or the surrounding soft tissue. In addition, the ability to selectively interrupt the innervation to the facial muscles could help differentiate masticatory myogenous pain from odontogenic pain by isolating the source of pain when encountering patients with difficulty localizing their source of pain (42).
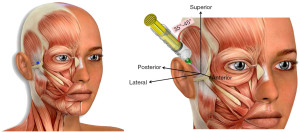
Serendipitously, we observed that pain relief from TMNB was often sustained for a period of weeks-months, far outlasting the duration of the local anesthetic itself (clinical observation of authors). This presented the possibility that the TMNB may have therapeutic value in relieving pain from the masseter and/or the temporalis muscles. The mechanism for TMNB’s prolonged pain relief is yet to be uncovered; we speculate that the innervation to the masseter and temporalis being mixed (sensory and motor), administration of the local anesthetic affects the motor activity of the muscles. This may, in turn, interrupt the ‘pain co-contraction pain’ cycle implicated in chronic pain, thereby relieving pain for longer durations of time than the action of the local anesthetic action.
While the mechanism for TNMB-mediated sustained pain relief is yet to be uncovered, data corroborates its efficacy in relieving chronic myofascial pain of masseteric origin (37,39). The effectiveness of pain relief from TMNB is comparable to Trigger point injections for up to six months (37). The significance of this is two-fold- one, the ability to identify trigger points requires specialized training that is not available to general dentists. As a result, patients with chronic masticatory myofascial pain are often misdiagnosed or subjected to multiple referrals and delayed care. The TMNB is easy to administer and requires no additional armamentarium beyond the dental anesthetic and syringe the general practitioner has ready access to. Second, it overrides the need to identify the active trigger point/s, potentially transforming masticatory myofascial pain into a condition that the general practitioner can readily diagnose and treat.
The key concern that needs to be addressed then is its safety. Over the last seven years that the TMNB has been in clinical use, there have been no reports of any adverse effects, short-term or lasting, from its use (manuscript in preparation) (43). Since the dental anesthetic is used on a routine basis globally, its safety is well-established.
The TMNB as a ‘sorting hat’ of mTMD into peripheral vs. central phenomena?
We speculate that a peripheral intervention such as the TMNB may be exceptionally effective in the assessment or management of masticatory myofascial pain (mTMD) when the dominant mechanism is local and help identify/delineate those who may have a central mechanism for their myofascial pain by perhaps their poor treatment response to TMNB. This may be critical in appropriately identifying those patients in need of more escalated, interdisciplinary care. There is no data to support this viewpoint, and it is purely hypothetical. However, we conclude that such research may well be warranted by the complexity and heterogeneity of patient presentation in mTMD.
Strengths and limitations
This manuscript critically appraises the literature to present a novel perspective on the management of mTMD and recommends the use of the TMNB as a possible intervention. The TMNB is simple and feasible to administer and is well-tolerated by the patient. However, it should be borne in mind that this discussion is speculative and this approach requires validation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mythili Kalladka) for the series “Orofacial Pain: Diagnostic and Therapeutic Topicals, Nerve Blocks and Trigger Point Injection” published in Journal of Oral and Maxillofacial Anesthesia. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://joma.amegroups.com/article/view/10.21037/joma-22-14/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://joma.amegroups.com/article/view/10.21037/joma-22-14/coif). The series “Orofacial Pain: Diagnostic and Therapeutic Topicals, Nerve Blocks and Trigger Point Injection” was commissioned by the editorial office without any funding or sponsorship. GS declares that granted with institutional support, and presented CE and received payment (Dec 2020) from Physicians’ Education Resource®, LLC (PER®) for CME program, titled Clinical Consultations™: Improving the Coordination of Care Between Dentists, Oral Surgeons, and Osteoporosis Specialists to Reduce the Risk of ONJ. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Von Korff M, Dworkin SF, Le Resche L, et al. An epidemiologic comparison of pain complaints. Pain 1988;32:173-83. [Crossref] [PubMed]
- Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain 2008;22:317-22. [PubMed]
- Bair E, Brownstein NC, Ohrbach R, et al. Study protocol, sample characteristics, and loss to follow-up: the OPPERA prospective cohort study. J Pain 2013;14:T2-19. [Crossref] [PubMed]
- Duarte FCK, West DWD, Linde LD, et al. Re-Examining Myofascial Pain Syndrome: Toward Biomarker Development and Mechanism-Based Diagnostic Criteria. Curr Rheumatol Rep 2021;23:69. [Crossref] [PubMed]
- Fricton J. Myofascial Pain: Mechanisms to Management. Oral Maxillofac Surg Clin North Am 2016;28:289-311. [Crossref] [PubMed]
- Cao QW, Peng BG, Wang L, et al. Expert consensus on the diagnosis and treatment of myofascial pain syndrome. World J Clin Cases 2021;9:2077-89. [Crossref] [PubMed]
- Prodoehl J, Kraus S, Klasser GD, et al. Temporomandibular disorder content in the curricula of physical therapist professional programs in the United States. Cranio 2020;38:376-88. [Crossref] [PubMed]
- Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:6-27. [Crossref] [PubMed]
- Bron C, Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep 2012;16:439-44. [Crossref] [PubMed]
- NIH. Quantitative Evaluation of Myofascial Tissues: Potential Impact for Musculoskeletal Pain Research. 2020.
- Vardeh D, Mannion RJ, Woolf CJ. Toward a Mechanism-Based Approach to Pain Diagnosis. J Pain 2016;17:T50-69. [Crossref] [PubMed]
- Jafri MS. Mechanisms of Myofascial Pain. Int Sch Res Notices 2014;2014:523924. [Crossref] [PubMed]
- Gerwin RD. Myofascial Trigger Point Pain Syndromes. Semin Neurol 2016;36:469-73. [Crossref] [PubMed]
- Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons' integrated hypothesis of trigger point formation. Curr Pain Headache Rep 2004;8:468-75. [Crossref] [PubMed]
- Woźniak K, Lipski M, Lichota D, et al. Muscle fatigue in the temporal and masseter muscles in patients with temporomandibular dysfunction. Biomed Res Int 2015;2015:269734. [Crossref] [PubMed]
- Stecco A, Gesi M, Stecco C, et al. Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep 2013;17:352. [Crossref] [PubMed]
- Fillingim RB, Slade GD, Greenspan JD, et al. Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: findings from the OPPERA study. Pain 2018;159:2403-13. [Crossref] [PubMed]
- Fernández-de-las-Peñas C, Dommerholt J. Myofascial trigger points: peripheral or central phenomenon? Curr Rheumatol Rep 2014;16:395. [Crossref] [PubMed]
- Ji RR, Nackley A, Huh Y, et al. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018;129:343-66. [Crossref] [PubMed]
- Maixner W, Diatchenko L, Dubner R, et al. Orofacial pain prospective evaluation and risk assessment study--the OPPERA study. J Pain 2011;12:T4-11.e1-2.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Board on Health Sciences Policy; Committee on Temporomandibular Disorders (TMDs): From Research Discoveries to Clinical Treatment, Yost O, Liverman CT, English R, et al. editors. Temporomandibular Disorders: Priorities for Research and Care. Washington (DC): National Academies Press (US); 2020.
- Fillingim RB, Ohrbach R, Greenspan JD, et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 2011;12:T46-60. [Crossref] [PubMed]
- Bair E, Gaynor S, Slade GD, et al. Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: the OPPERA study. Pain 2016;157:1266-78. [Crossref] [PubMed]
- Kalladka M, Young A, Khan J. Myofascial pain in temporomandibular disorders: Updates on etiopathogenesis and management. J Bodyw Mov Ther 2021;28:104-13. [Crossref] [PubMed]
- Al-Moraissi EA, Wolford LM, Ellis E 3rd, et al. The hierarchy of different treatments for arthrogenous temporomandibular disorders: A network meta-analysis of randomized clinical trials. J Craniomaxillofac Surg 2020;48:9-23. [Crossref] [PubMed]
- Feng J, Luo M, Ma J, et al. The treatment modalities of masticatory muscle pain a network meta-analysis. Medicine (Baltimore) 2019;98:e17934. [Crossref] [PubMed]
- Al-Moraissi EA, Farea R, Qasem KA, et al. Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: network meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg 2020;49:1042-56. [Crossref] [PubMed]
- Noma N, Watanabe Y, Shimada A, et al. Effects of cognitive behavioral therapy on orofacial pain conditions. J Oral Sci 2020;63:4-7. [Crossref] [PubMed]
- Fernandes G, Gonçalves DAG, Conti P. Musculoskeletal Disorders. Dent Clin North Am 2018;62:553-64. [Crossref] [PubMed]
- Calixtre LB, Moreira RF, Franchini GH, et al. Manual therapy for the management of pain and limited range of motion in subjects with signs and symptoms of temporomandibular disorder: a systematic review of randomised controlled trials. J Oral Rehabil 2015;42:847-61. [Crossref] [PubMed]
- Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther 2009;14:531-8. [Crossref] [PubMed]
- Al-Moraissi EA, Alradom J, Aladashi O, et al. Needling therapies in the management of myofascial pain of the masticatory muscles: A network meta-analysis of randomised clinical trials. J Oral Rehabil 2020;47:910-22. [Crossref] [PubMed]
- Hammi C, Schroeder JD, Yeung B. Trigger Point Injection. Treasure Island (FL): StatPearls Publishing; July 25, 2022.
- Dunning J, Butts R, Mourad F, et al. Dry needling: a literature review with implications for clinical practice guidelines. Phys Ther Rev 2014;19:252-65. [Crossref] [PubMed]
- Serritella E, Galluccio G, Impellizzeri A, et al. Comparison of the Effectiveness of Three Different Acupuncture Methods for TMD-Related Pain: A Randomized Clinical Study. Evid Based Complement Alternat Med 2021;2021:1286570. [Crossref] [PubMed]
- Karamanlioglu DS, Geler Kulcu D, Ozturk G, et al. Effectiveness of pregabalin treatment for trigger points in patients with comorbid myofascial pain syndrome and fibromyalgia syndrome: a randomized controlled trial. Somatosens Mot Res 2021;38:327-32. [Crossref] [PubMed]
- Ananthan S, Kanti V, Zagury JG, et al. The effect of the twin block compared with trigger point injections in patients with masticatory myofascial pain: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol 2020;129:222-8. [Crossref] [PubMed]
- Ananthan S, Subramanian G, Patel T, et al. The twin block injection: an adjunctive clinical aid for the management of acute arthrogenous temporomandibular joint dysfunction. Quintessence Int 2020;51:330-3. [PubMed]
- Kanti V, Ananthan S, Subramanian G, et al. Efficacy of the twin block, a peripheral nerve block for the management of chronic masticatory myofascial pain: A case series. Quintessence Int 2017; Epub ahead of print. [Crossref] [PubMed]
- Quek S, Young A, Subramanian G. The twin block: a simple technique to block both the masseteric and the anterior deep temporal nerves with one anesthetic injection. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:e65-7. [Crossref] [PubMed]
- Quek SYP, Gomes-Zagury J, Subramanian G. Twin Block in Myogenous Orofacial Pain: Applied Anatomy, Technique Update, and Safety. Anesth Prog 2020;67:103-6. [Crossref] [PubMed]
- Quek SYP, Kalladka M, Kanti V, et al. A new adjunctive tool to aid in the diagnosis of myogenous temporomandibular disorder pain originating from the masseter and temporalis muscles: Twin-block technique. J Indian Prosthodont Soc 2018;18:181-5. [Crossref] [PubMed]
- Makhija D, Quek SYP, Subramanian G. The Twin Block Safely and Effectively Alleviates Masticatory Myogenous Pain. 2021 IADR/AADR/CADR General Session (Virtual Experience). 2021.
Cite this article as: Subramanian G, Ananthan S, Zagury JG, Quek SYP. Pragmatic management of myogenous temporomandibular disorder—a narrative review. J Oral Maxillofac Anesth 2022;1:35.


