
Antivitamin action of nitrous oxide in OMF surgery—a narrative review
Introduction
Background
As an aid to dentistry, nitrous oxide was the first inhaled anesthetic to reach public attention. Now, many, perhaps most, inhaled anesthetics for OMF surgery include the gas. Its antivitamin action is generally disregarded. Accordingly, the primary objective of this review is to illuminate this often subtle but detectable toxicity.
The gas was discovered in 1772 in bubbles arising from the action of aqueous nitric acid on metals. In 1799, Humphry Davy found the pure gas to be nonirritating and intoxicating (1,2). He staged popular demonstrations at the Royal Institution in London, and the substance became known as “laughing gas” (1).
Medical student Gardner Quincy Colton staged laughing gas shows in America (3,4). After witnessing a Colton show in 1844, American dentist Horace Wells proposed nitrous oxide for painless tooth extractions (5). Wells failed to convince witnesses at the Harvard Medical School that nitrous is an effective anesthetic (5), but his partner William T.G. Morton successfully introduced relatively potent diethyl ether vapor there (6). The landmark Morton demonstration was for an OMF surgery, the excision of a jaw tumor, on October 16, 1846. Despite the triumph of Morton on Ether Day, Colton clung to nitrous and became the busiest painless dentist in Manhattan (4).
The last diethyl ether anesthetic at Harvard was in 1978, but nitrous oxide is a frequent anesthetic agent today. However, the gas interferes with the function of vitamin B12. It covalently inactivates the enzyme methionine synthase, one of the two important B12 enzymes in humans.
Rationale and knowledge gap
We consider the mechanism and the clinical ramifications of the antivitamin phenomenon.
Objective
We consider situations in which nitrous oxide toxicity may be appreciable.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://joma.amegroups.com/article/view/10.21037/joma-22-21/rc).
Methods
By means of the PubMed search engine of the United States National Library of Medicine, the MEDLINE database of references and abstracts on life sciences and biomedical topics was searched for information in the English language from 1956 to 2022 (Table 1). Full articles were accessed via the Countway Library of the Harvard Medical School. SANRA criteria were guidelines.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1 February 2022 to 8 August 2022 |
| Databases and other sources searched | MEDLINE |
| Search terms used | Cobalamin; DNA; folinic acid; homocysteine; methionine; myelin; nitrous, oxide; pernicious anemia; pyridoxal; spongiform; tetrahydrofolic acid; vitamin B12 |
| Timeframe | 1956 to 2022 |
| Inclusion criteria | Primary reports favored; English language |
| Selection process | Reviewer discretion |
Findings
Antivitamin action
Evidence of antagonism of vitamin B12 appeared in 1956 (7). Tetanus victims requiring mechanical ventilation, sedation, and analgesia were ventilated with 50% N2O for several days, whereupon peripheral blood smears became marked by megaloblasts (7). Megaloblasts are large, nucleated precursors of red corpuscles. They do not normally circulate but are particularly prominent in so-called pernicious anemia (7). Pernicious anemia was recognized in the 1800s as an anemia that was usually fatal within three years of its symptoms. The disease led to the life-saving discovery of vitamin B12 in the 1900s. Accordingly, the megaloblastosis of nitrous oxide exposure pointed to the deficient function of vitamin B12 (7).
Methionine synthase
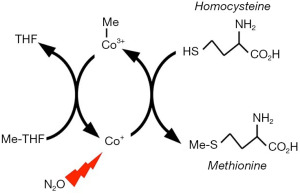
There are two vitamin B12-dependent enzymes in humans. They are methionine synthase (Figure 1) and methylmalonyl-CoA mutase. The first of these is covalently inactivated by N2O. The synthase reaction proceeds according to Equation [1].
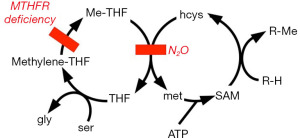
Methionine is one of the 20 amino acids that commonly comprise protein. It is an “essential” amino acid, required in the diet. Why then is a methionine-synthesizing enzyme important in human metabolism? Methionine is an S-methyl compound and is an important source of one-carbon (methyl) groups in biosynthesis (Figure 2). Many methylations in metabolism require methionine to be consumed and then rebuilt by methionine synthase in the so-called methionine cycle. For instance, norepinephrine is methylated to epinephrine by an N-methyltransferase, and catecholamines are inactivated upon methylation by an O-methyltransferase. Other examples of methyl groups arising from methionine are those of acetylcholine and choline-containing lipids. The lecithin of bile is a choline-containing lipid (which is historically why the word acetylcholine sounds like cholecystectomy). Another choline-derived lipid is sphingomyelin, a major component of myelin sheaths of nerves. A methylation that indirectly involves methionine synthase is the process by which uracil becomes the thymine of DNA (Figure 3).
The vitamin B12 molecule contains a cobalt ion and is thus also known as cobalamin. In vitamin pills, the molecule is stabilized by a functionally inactive cyanide group and is also called cyanocobalamin. The cobalt atom of cyanocobalamin is in an oxidized trivalent state (Co3+) and is not further oxidized by nitrous oxide. However, in the catalytic active site of methionine synthase, the cyanide-free cobalt functions in the unstable Co1+ state (Figure 1). Unshielded by the protein, highly reactive univalent cobalt can reduce water to hydrogen gas. At anesthetic concentrations, N2O can gain access to the reactive cobalt atom. The N2O is reduced by one-electron to a hydroxyl free radical (HO·) that then inactivates a vital part of the enzyme (8). The hydroxyl radical arises according to Equation [2].
The damaged enzyme cannot be repaired, and recovery requires synthesis of new enzyme molecules from fresh amino acids, a process with a half-time of days in humans (8,9). The rate of inactivation of the enzyme is not precisely known, and the process might not be monophasic in vivo. Probably because of high metabolic rates, rodents are relatively sensitive. Liver biopsies in humans show that about half of the enzyme is inactivated by 50–70% nitrous in a few hours (9).
Methylmalonyl-CoA mutase is involved in the catabolism of fatty acids and amino acids that give rise to propionic acid, CH3-CH2-COOH. The mutase does not react directly with N2O. However, prolonged exposures to N2O may indirectly reduce the mutase level since cobalamin is consumed in methionine synthase reaction with N2O. The cofactor for the mutase is a form of vitamin B12 known as adenosylcobalamin, and it is not known if N2O affects the adenosylation of cobalamin.
Consequences of inactivation of methionine synthase
There are at least three consequences of the inactivation of methionine synthase. They are impaired DNA synthesis, neuropathy, and elevation of plasma homocysteine.
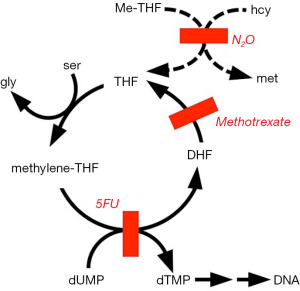
DNA synthesis is inhibited because methionine synthase is involved in the incorporation of thymine into DNA (Figure 3). Dietary thymine is poorly utilized in the synthesis of DNA. Instead, a deoxyuridine nucleotide is methylated to a thymidine one. Impaired DNA synthesis after N2O exposure has been demonstrated in humans by means of an ex-vivo cellular assay known as the deoxyuridine suppression test, in which deoxyuridine inhibits incorporation of radiolabeled thymidine into DNA (10). Other evidence includes cases of megaloblastosis and macrocytic anemia (11).
As in the case of untreated pernicious anemia, N2O has been associated with cases of demyelination of the spinal cord (11-17). N2O has been associated with other neuropathies (18-21) and psychiatric problems (22-27) in patients at increased risk of toxicity. Demyelination may arise because methionine synthase provides methyl groups for the sphingomyelin lipid molecule (Figure 2). The enzyme also provides methyl groups for acetylcholine, epinephrine, and O-methyl catecholamines.
The in vitro assay of methionine synthase is cumbersome and is best suited to biopsy specimens of solid tissues. Accordingly, the kinetics of enzyme inactivation and regeneration are known only roughly in humans. However, acute metabolic action of nitrous oxide is readily detected as an increase in plasma levels of homocysteine (28-34). Homocysteine is the precursor of methionine in the synthase reaction (Figure 1).
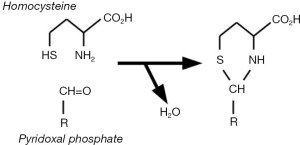
Though homocysteine is an essential metabolic intermediate, its elevated concentration is toxic. For one thing, the molecule spontaneously forms a stable covalent derivative of pyridoxal phosphate, the enzymatically active form of the B6 vitamin pyridoxine (Figure 4) (35,36). Adduct formation depletes pyridoxal phosphate (the enzyme-cofactor form of pyridoxine), and the adduct is an inhibitor of pyridoxal phosphate dependent enzymes. These include serine hydroxymethyltransferase, which converts tetrahydrofolate into methylene-tetrahydrofolate (Figures 2,3). Consequently, impaired function of vitamin B12 disrupts function of the vitamins B6 (pyridoxine) and B9 (folic acid).
Another mechanism of homocysteine toxicity involves its metabolism to a chemically reactive product. The ATP-utilizing synthetase that normally attaches methionine to tRNA also attaches homocysteine to tRNA. Methionyl-tRNA is used for protein synthesis, but homocysteinyl-tRNA is unstable (Figure 5). It yields a cyclic thioester known as homocysteine thiolactone (37). The thiolactone can nonspecifically attach to cellular constituents, and it specifically blocks the active site of lysyl oxidase, an enzyme involved in the cross-linking of strands of collagen and elastin in blood vessels and other connective tissues (38).

The inactivation of lysyl oxidase may contribute to the atherogenic action of homocysteine (39). However, that and prothrombotic actions might involve reactions of the thiolactone and/or homocysteine with other targets (20,39-42). For instance, they affect vascular endothelium. In particular, they inhibit endothelial production of nitric oxide (NO), a two-atom gas molecule that is not to be confused with the three-atom anesthetic nitrous oxide (N2O). The subject of a Nobel Prize in 1997, NO inhibits platelet adhesion and aggregation, and it dilates blood vessels. In addition to inhibiting endothelial NO synthesis, sulfur compounds such as homocysteine react directly with and thus scavenge NO (28).
Cardiovascular complications have been ascribed to chronic hyperhomocysteinemia (39,40). However, the ENIGMA-II study demonstrated the long-term safety of one-time nitrous oxide administration as part of the anesthetic regimen in noncardiac surgical patients with known or suspected coronary artery disease (43,44).
Risk factors for N2O toxicity
Serious metabolic problems are rarely seen when N2O is used as an anesthetic. Risk factors for toxicity include prolonged exposure (24,45,46); methotrexate and related drug therapy (47-49); genetic problems such as deficiency of methylene-tetrahydrofolate reductase; pernicious anemia (7,11); and, presumably, dietary deficiency of vitamins.
Prolonged exposure to N2O can include recreational abuse of the intoxicating drug (24) as well as occupational exposure (45). Recreational exposure can involve small cylinders of the gas known as whippits or whippets. These are intended for use in the whipping of cream for culinary purposes. The agent is discharged into party balloons in decompression for inhalation. Occupational exposure is most likely to occur near patients receiving the gas by loose-fitting masks. The inactivation of methionine synthase persists following discontinuation of an anesthetic, so toxicity can arise from either lengthy anesthetics or else repetitive exposure (46).
Methotrexate inhibits dihydrofolate reductase, and N2O inactivates methionine synthase. Both enzymes produce tetrahydrofolate, so the combined inhibitors can profoundly impair the availability of tetrahydrofolate to participate in the synthesis of DNA. In laboratory experiments with rodents, N2O is easily demonstrated to convert nonlethal doses of methotrexate into lethal doses (47). Potentiation of methotrexate by N2O has also been encountered in humans (48,49).
Any genetic defect in folate or vitamin B12 metabolism might predispose to N2O toxicity. One example is deficiency of methylene-tetrahydrofolate reductase (12,50-53). As exemplified by pernicious anemia, acquired problems in B12 or folate status can also facilitate N2O toxicity (54). Nutritional depletion of protein and/or vitamins would also increase the likelihood of metabolic complications of N2O (55-57). Preoperative supplementation of vitamin B12 reduces postoperative elevation of homocysteine (35,58), but the effect is variable (59).
Treatment/rescue
A lesson from pernicious anemia was that vitamin B12 deficiency can be palliated by nutritional agents. For instance, the anemia of early pernicious anemia could be masked by doses of folic acid. Unfortunately, the neuropathy of the disease proceeded in spite of folate supplementation. One strategy has been to provide supplemental tetrahydrofolate. However, that molecule is significantly unstable in the presence of oxygen. Accordingly, tetrahydrofolate is given in the form of a relatively stable derivative known as folinic acid (60,61). Chemically, that is N-formyltetrahydrofolate. The molecule was discovered in 1948 as an essential growth factor acid for the bacterium Leuconostoc citrovorum, and it has thus been called citrovorum factor and also leucovorin. It was clinically introduced for rescue from methotrexate. It permits some DNA synthesis in the presence of inhibitors of dihydrofolate reductase or methionine synthase (Figure 3). However, full rescue would require its recycling in the body, and the recycling is inhibited by the presence of the mentioned enzyme inhibitors.
Exogenous thymine base is poorly utilized for DNA synthesis (62-65), but the nucleoside thymidine merits investigation as a rescue agent in view of benefit seen in pernicious anemia (66). Since sphingomyelin of myelin is a choline ester, it would be of interest to examine dietary choline for prevention of N2O-induced demyelination (67).
Dietary supplementation with exogenous methionine and betaine (N,N,N-trimethylglycine) can palliate errors in cobalamin metabolism (68). The most common case is cobalamin C disorder, in which dietary cyanocobalamin and other forms of vitamin B12 are not converted to the enzymatically active form for methionine synthetase (nor that for methylmalonyl-CoA mutase). When a patient with early onset of the disorder consistently took methionine and betaine, methionine levels were raised to normal levels (69). Normalizing methionine levels may prevent CNS sequelae associated with cobalamin C disorder, including subacute combined degeneration (SCD) of the spinal cord (69). However, these findings have not been well studied in humans. Methionine supplementation is protective against SCD in monkeys and pigs exposed to nitrous oxide (70,71).
Because the cobalamin cofactor is covalently consumed in the reaction of methionine synthase with N2O, administration of vitamin B12 is plausibly beneficial before or after administration of N2O. Vitamin B12 is clearly indicated if its deficiency is clinically apparent.
Theoretical considerations
In view of the strong oxidation-reduction potential of the Co1+ center of methionine synthase, other oxidants in addition to N2O might also react with the enzyme. The one drug in addition to N2O that is known to inactivate methionine synthase is chloroform (72-75). Inactivation of methionine synthesis by chloroform was demonstrated in a B12-dependent E. coli strain after chloroform and related molecules were noted to block B12-dependent methane biosynthesis in the rumen-dwelling bacteria of cattle (76-78). It is intriguing to wonder if N2O or chloroform might have exploitable antibiotic activity against pathogenic B12-dependent microbes. For instance, malaria parasites carry a B12-dependent methionine synthase (79). Presently, there are no examples of nitrous oxide as an antimicrobial drug, and its inhalation in anesthetic doses precludes hyperoxia as an anti-anaerobe strategy (46,80).
N2O is selectively toxic to dividing neoplastic cells (46,78-85). In view of the permeability of skin to inhaled anesthetics (86), it would be interesting to evaluate the gas for topical therapy of cutaneous lesions.
Conclusions
Inhaled N2O is a useful and generally safe anesthetic agent. However, it antagonizes the function of vitamin B12, a factor that participates in DNA synthesis, nerve myelination, and homocysteine scavenging. Accordingly, it is prudent to avoid prolonged or repetitive N2O exposures to anesthetic/sedative doses. Alternative anesthetic agents should be considered in cases of neuropathy and in cases of relevant metabolic impairments such as those of concurrent methotrexate therapy; genetic deficiency of methylene-tetrahydrofolate reductase; pernicious anemia; and poor nutrition. However, neither routine screening for vitamin B12 status, nor supplementation with vitamin B12, is likely to have clinical value for one-time anesthetic exposures of a few hours or less.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jingping Wang and Christopher Fanelli) for the series “Opioid-free Anesthesia and Opioid-sparing Anesthesia in OMF Surgery” published in Journal of Oral and Maxillofacial Anesthesia. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://joma.amegroups.com/article/view/10.21037/joma-22-21/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://joma.amegroups.com/article/view/10.21037/joma-22-21/coif). The series “Opioid-free Anesthesia and Opioid-sparing Anesthesia in OMF Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davy H. Researches, Chemical and Philosophical; Chiefly Concerning Nitrous Oxide: Or Dephlogisticated Nitrous Air, and its Respiration. London, UK: J. Johnson; 1800.
- Alston TA. Early misconceptions about nitrous oxide, an "invigorating" asphyxiant. J Clin Anesth 2010;22:59-63. [Crossref] [PubMed]
- Smith GB, Hirsch NP. Gardner Quincy Colton: pioneer of nitrous oxide anesthesia. Anesth Analg 1991;72:382-91. [Crossref] [PubMed]
- Yang QH, Alston TA, Phineas T. Barnum, Gardner Q. Colton, and Painless Parker Were Kindred Princes of Humbug. J Anesth Hist 2019;5:13-21. [Crossref] [PubMed]
- Haridas RP. Horace wells' demonstration of nitrous oxide in Boston. Anesthesiology 2013;119:1014-22. [Crossref] [PubMed]
- Fenster JM. Ether Day: The Strange Tale of America's Greatest Medical Discovery and the Haunted Men Who Made It. New York, NY: HarperCollins; 2002.
- Lassen HC, Henriksen E, Neukirch F, et al. Treatment of tetanus; severe bone-marrow depression after prolonged nitrous-oxide anaesthesia. Lancet 1956;270:527-30. [PubMed]
- Drummond JT, Matthews RG. Nitrous oxide inactivation of cobalamin-dependent methionine synthase from Escherichia coli: characterization of the damage to the enzyme and prosthetic group. Biochemistry 1994;33:3742-50. [Crossref] [PubMed]
- Koblin DD, Waskell L, Watson JE, et al. Nitrous oxide inactivates methionine synthetase in human liver. Anesth Analg 1982;61:75-8. [Crossref] [PubMed]
- Amess JA, Burman JF, Rees GM, et al. Megaloblastic haemopoiesis in patients receiving nitrous oxide. Lancet 1978;2:339-42. [Crossref] [PubMed]
- Hogue CW Jr, Perese D, Vacanti CA, et al. Potential toxicity from prolonged anesthesia: a case report of a thirty-hour anesthetic. J Clin Anesth 1990;2:183-7. [Crossref] [PubMed]
- Yu M, Qiao Y, Li W, et al. Analysis of clinical characteristics and prognostic factors in 110 patients with nitrous oxide abuse. Brain Behav 2022;12:e2533. [Crossref] [PubMed]
- Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: A systematic review of the case literature. Am J Addict 2016;25:358-69. [Crossref] [PubMed]
- Check L, Abdelsayed N, Figueroa G, et al. Subacute Combined Degeneration of the Cervical Spine Secondary to Inhaled Nitrous-Oxide-Induced Cobalamin Deficiency. Cureus 2022;14:e21214. [Crossref] [PubMed]
- Agarwal P, Khor SY, Do S, et al. Recreational Nitrous Oxide-Induced Subacute Combined Degeneration of the Spinal Cord. Cureus 2021;13:e19377. [Crossref] [PubMed]
- Cao J, Ran L, Liu C, et al. Serum copper decrease and cerebellar atrophy in patients with nitrous oxide-induced subacute combined degeneration: two cases report. BMC Neurol 2021;21:471. [Crossref] [PubMed]
- Berling E, Fargeot G, Aure K, et al. Nitrous oxide-induced predominantly motor neuropathies: a follow-up study. J Neurol 2022;269:2720-6. [Crossref] [PubMed]
- Richardson PG. Peripheral neuropathy following nitrous oxide abuse. Emerg Med Australas 2010;22:88-90. [PubMed]
- Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol 2015;15:207-9. [Crossref] [PubMed]
- Kuipers RS, Paes AJ, Amoroso G, et al. Thrombosis within the left anterior descending coronary artery and left ventricle after high-dose nitrous oxide use. BMJ Case Rep 2022;15:e248281. [Crossref] [PubMed]
- Sluyts Y, Vanherpe P, Amir R, et al. Vitamin B(12) deficiency in the setting of nitrous oxide abuse: diagnostic challenges and treatment options in patients presenting with subacute neurological complications. Acta Clin Belg 2022;77:955-61. [Crossref] [PubMed]
- Singh SK, Misra UK, Kalita J, et al. Nitrous oxide related behavioral and histopathological changes may be related to oxidative stress. Neurotoxicology 2015;48:44-9. [Crossref] [PubMed]
- Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide "whippit" abuse presenting with cobalamin responsive psychosis. J Med Toxicol 2006;2:71-4. [Crossref] [PubMed]
- Levine J, Chengappa KN. Exposure to nitrous oxide may be associated with high homocysteine plasma levels and a risk for clinical depression. J Clin Psychopharmacol 2007;27:238-9. [Crossref] [PubMed]
- Pedersen OB, Hvas AM, Grove EL A. 19-Year-Old Man with a History of Recreational Inhalation of Nitrous Oxide with Severe Peripheral Neuropathy and Central Pulmonary Embolism. Am J Case Rep 2021;22:e931936. [Crossref] [PubMed]
- Xiang Y, Li L, Ma X, et al. Recreational Nitrous Oxide Abuse: Prevalence, Neurotoxicity, and Treatment. Neurotox Res 2021;39:975-85. [Crossref] [PubMed]
- Wu G, Wang S, Wang T, et al. Neurological and Psychological Characteristics of Young Nitrous Oxide Abusers and Its Underlying Causes During the COVID-19 Lockdown. Front Public Health 2022;10:854977. [Crossref] [PubMed]
- Myles PS, Chan MT, Kaye DM, et al. Effect of nitrous oxide anesthesia on plasma homocysteine and endothelial function. Anesthesiology 2008;109:657-63. [Crossref] [PubMed]
- Myles PS, Chan MT, Leslie K, et al. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. Br J Anaesth 2008;100:780-6. [Crossref] [PubMed]
- Nagele P, Tallchief D, Blood J, et al. Nitrous oxide anesthesia and plasma homocysteine in adolescents. Anesth Analg 2011;113:843-8. [Crossref] [PubMed]
- Cascella M, Arcamone M, Morelli E, et al. Multidisciplinary approach and anesthetic management of a surgical cancer patient with methylene tetrahydrofolate reductase deficiency: a case report and review of the literature. J Med Case Rep 2015;9:175. [Crossref] [PubMed]
- Kiasari AZ, Firozian A, Baradari AG, et al. The effect of vitamin B12 infusion on prevention of nitrous oxide-induced homocysteine increase: a double-blind randomized control trial. Oman Med J 2014;29:194-7. [Crossref] [PubMed]
- Pichardo D, Luginbuehl IA, Shakur Y, et al. Effect of nitrous oxide exposure during surgery on the homocysteine concentrations of children. Anesthesiology 2012;117:15-21. [Crossref] [PubMed]
- Nagele P, Zeugswetter B, Eberle C, et al. A common gene variant in methionine synthase reductase is not associated with peak homocysteine concentrations after nitrous oxide anesthesia. Pharmacogenet Genomics 2009;19:325-9. [Crossref] [PubMed]
- Pestaña A, Sandoval IV, Sols A. Inhibition by homocysteine of serine dehydratase and other pyridoxal 5'-phosphate enzymes of the rat through cofactor blockage. Arch Biochem Biophys 1971;146:373-9. [Crossref] [PubMed]
- Głowacki R, Stachniuk J, Borowczyk K, et al. Quantification of homocysteine and cysteine by derivatization with pyridoxal 50 -phosphate and hydrophilic interaction liquid chromatography. Anal Bioanal Chem 2016;408:1935-41. [Crossref] [PubMed]
- Jakubowski H. Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr 2000;130:377S-81S. [Crossref] [PubMed]
- Liu G, Nellaiappan K, Kagan HM. Irreversible inhibition of lysyl oxidase by homocysteine thiolactone and its selenium and oxygen analogues. Implications for homocystinuria. J Biol Chem 1997;272:32370-7. [Crossref] [PubMed]
- Bełtowski J. Protein homocysteinylation: a new mechanism of atherogenesis? Postepy Hig Med Dosw (Online) 2005;59:392-404. [PubMed]
- Yilmaz N. Relationship between paraoxonase and homocysteine: crossroads of oxidative diseases. Arch Med Sci 2012;8:138-53. [Crossref] [PubMed]
- Oulkadi S, Peters B, Vliegen AS. Thromboembolic complications of recreational nitrous oxide (ab)use: a systematic review. J Thromb Thrombolysis 2022;54:686-95. [Crossref] [PubMed]
- de Valck L, Defelippe VM, Bouwman NAMG. Cerebral venous sinus thrombosis: a complication of nitrous oxide abuse. BMJ Case Rep 2021;14:e244478. [Crossref] [PubMed]
- Leslie K, Myles PS, Kasza J, et al. Nitrous oxide and serious long-term morbidity and mortality in evaluation of nitrous oxide in the gas mixture for anaesthesia (ENIGMA)-II trial. Anesthesiology 2015;123:1267-80. [Crossref] [PubMed]
- Myles PS, Leslie K, Chan MT, et al. The safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery (ENIGMA-II): a randomized, single-blind trial. Lancet 2014;384:1446-54. [Crossref] [PubMed]
- Krajewski W, Kucharska M, Pilacik B, et al. Impaired vitamin B12 metabolic status in healthcare workers occupationally exposed to nitrous oxide. Br J Anaesth 2007;99:812-8. [Crossref] [PubMed]
- Chen Y, Liu X, Cheng CH, et al. Leukocyte DNA damage and wound infection after nitrous oxide administration: a randomized controlled trial. Anesthesiology 2013;118:1322-31. [Crossref] [PubMed]
- Ermens AA, Schoester M, Spijkers LJ, et al. Toxicity of methotrexate in rats preexposed to nitrous oxide. Cancer Res 1989;49:6337-41. [PubMed]
- Fiskerstrand T, Ueland PM, Refsum H. Folate depletion induced by methotrexate affects methionine synthase activity and its susceptibility to inactivation by nitrous oxide. J Pharmacol Exp Ther 1997;282:1305-11. [PubMed]
- Löbel U, Trah J, Escherich G. Severe neurotoxicity following intrathecal methotrexate with nitrous oxide sedation in a child with acute lymphoblastic leukemia. Pediatr Blood Cancer 2015;62:539-41. [Crossref] [PubMed]
- Selzer RR, Rosenblatt DS, Laxova R, et al. Adverse effect of nitrous oxide in a child with 5,10-methylenetetrahydrofolate reductase deficiency. N Engl J Med 2003;349:45-50. [Crossref] [PubMed]
- Gerges FJ, Dalal AR, Robelen GT, et al. Anesthesia for cesarean section in a patient with placenta previa and methylenetetrahydrofolate reductase deficiency. J Clin Anesth 2006;18:455-9. [Crossref] [PubMed]
- Nagele P, Brown F, Francis A, et al. Influence of nitrous oxide anesthesia, B-vitamins, and MTHFR gene polymorphisms on perioperative cardiac events: the vitamins in nitrous oxide (VINO) randomized trial. Anesthesiology 2013;119:19-28. [Crossref] [PubMed]
- Nagele P, Zeugswetter B, Wiener C, et al. Influence of methylenetetrahydrofolate reductase gene polymorphisms on homocysteine concentrations after nitrous oxide anesthesia. Anesthesiology 2008;109:36-43. [Crossref] [PubMed]
- Singer MA, Lazaridis C, Nations SP, et al. Reversible nitrous oxide-induced myeloneuropathy with pernicious anemia: case report and literature review. Muscle Nerve 2008;37:125-9. [Crossref] [PubMed]
- Ahn SC, Brown AW. Cobalamin deficiency and subacute combined degeneration after nitrous oxide anesthesia: a case report. Arch Phys Med Rehabil 2005;86:150-3. [Crossref] [PubMed]
- Felmet K, Robins B, Tilford D, et al. Acute neurologic decompensation in an infant with cobalamin deficiency exposed to nitrous oxide. J Pediatr 2000;137:427-8. [Crossref] [PubMed]
- Beltramello A, Puppini G, Cerini R, et al. Subacute combined degeneration of the spinal cord after nitrous oxide anaesthesia: role of magnetic resonance imaging. J Neurol Neurosurg Psychiatry 1998;64:563-4. [Crossref] [PubMed]
- Badner NH, Freeman D, Spence JD. Preoperative oral B vitamins prevent nitrous oxide-induced postoperative plasma homocysteine increases. Anesth Analg 2001;93:1507-10. table of contents. [Crossref] [PubMed]
- Rao LK, Francis AM, Wilcox U, et al. Pre-operative vitamin B infusion and prevention of nitrous oxide-induced homocysteine increase. Anaesthesia 2010;65:710-5. [Crossref] [PubMed]
- Amos RJ, Amess JA, Nancekievill DG, et al. Prevention of nitrous oxide-induced megaloblastic changes in bone marrow using folinic acid. Br J Anaesth 1984;56:103-7. [Crossref] [PubMed]
- Palmer AM, Kamynina E, Field MS, et al. Folate rescues vitamin B(12) depletion-induced inhibition of nuclear thymidylate biosynthesis and genome instability. Proc Natl Acad Sci U S A 2017;114:E4095-102. [Crossref] [PubMed]
- Plentl AA, Schoenheimer R. Studies in the metabolism of purines and pyrimidines by means of isotopic nitrogen. J Biol Chem 1944;153:203-17. [Crossref]
- Welch AD, Heinle RW. Hematopoietic agents in macrocytic anemias. Pharmacol Rev 1951;3:345-411. [PubMed]
- Spies TD, Frommeyer WB Jr. Anti-anemic properties of thymine. Blood 1946;1:185-8. [Crossref] [PubMed]
- Vilter RW, Horrigan D, Mueller JF, et al. Studies on the relationships of vitamin B12, folic acid, thymine, uracil and methyl group donors in persons with pernicious anemia and related megaloblastic anemias. Blood 1950;5:695-717. [Crossref] [PubMed]
- Hausmann K. Haemopoietic effect of thymidine in pernicious anaemia. Lancet 1951;1:329-30. [Crossref] [PubMed]
- Bennett MA. Some observations on the role of folic acid in utilization of homocystine by the rat. Science 1949;110:589. [Crossref] [PubMed]
- Christensen B, Guttormsen AB, Schneede J, et al. Preoperative methionine loading enhances restoration of the cobalamin-dependent enzyme methionine synthase after nitrous oxide anesthesia. Anesthesiology 1994;80:1046-56. [Crossref] [PubMed]
- Smith SE, Kinney HC, Swoboda KJ, et al. Subacute combined degeneration of the spinal cord in cbIC disorder despite treatment with B12. Mol Genet Metab 2006;88:138-45. [Crossref] [PubMed]
- Weir DG, Keating S, Molloy A, et al. Methylation deficiency causes vitamin B12-associated neuropathy in the pig. J Neurochem 1988;51:1949-52. [Crossref] [PubMed]
- Scott JM, Dinn JJ, Wilson P, et al. Pathogenesis of subacute combined degeneration: a result of methyl group deficiency. Lancet 1981;2:334-7. [Crossref] [PubMed]
- Alston TA. Inhibition of vitamin B12-dependent methionine biosynthesis by chloroform and carbon tetrachloride. Biochem Pharmacol 1991;42:R25-8. [Crossref] [PubMed]
- Alston TA. Inhibition of vitamin B12-dependent microbial growth by nitrous oxide. Life Sci 1991;48:1591-5. [Crossref] [PubMed]
- Alston TA. Chloroform, vitamin B12, and the tragic lives of Robert M. Glover and Horace Wells. Anaesthesia 2004;59:1147-8. [Crossref] [PubMed]
- Bhaskar H, Chaudhary R. Vitamin B12 Deficiency due to Chlorofluorocarbon: A Case Report. Case Rep Med 2010;2010:691563. [Crossref] [PubMed]
- Bauchop T. Inhibition of rumen methanogenesis by methane analogues. J Bacteriol 1967;94:171-5. [Crossref] [PubMed]
- Wood JM, Kennedy FS, Wolfe RS. The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B 12. Biochemistry 1968;7:1707-13. [Crossref] [PubMed]
- Alston TA. Don't have a cow! Fight global warming with CFC. Nature 2004;430:965. [Crossref] [PubMed]
- Krungkrai J, Webster HK, Yuthavong Y. Characterization of cobalamin-dependent methionine synthase purified from the human malarial parasite, Plasmodium falciparum. Parasitol Res 1989;75:512-7. [Crossref] [PubMed]
- Fleischmann E, Lenhardt R, Kurz A, et al. Nitrous oxide and risk of surgical wound infection: a randomised trial. Lancet 2005;366:1101-7. [Crossref] [PubMed]
- Kano Y, Sakamoto S, Sakuraya K, et al. Effect of nitrous oxide on human bone marrow cells and its synergistic effect with methionine and methotrexate on functional folate deficiency. Cancer Res 1981;41:4698-701. [PubMed]
- Ermens AA, Kroes AC, Schoester M, et al. Effect of cobalamin inactivation on folate metabolism of leukemic cells. Leuk Res 1988;12:905-10. [Crossref] [PubMed]
- Kroes AC, Ermens AA, Lindemans J, et al. Effects of 5-fluorouracil treatment of rat leukemia with concomitant inactivation of cobalamin. Anticancer Res 1986;6:737-42. [PubMed]
- Kroes AC, Lindemans J, Schoester M, et al. Enhanced therapeutic effect of methotrexate in experimental rat leukemia after inactivation of cobalamin (vitamin B12) by nitrous oxide. Cancer Chemother Pharmacol 1986;17:114-20. [Crossref] [PubMed]
- Young MJ, Alston TA. Does anesthetic technique influence cancer? J Clin Anesth 2012;24:1-2. [Crossref] [PubMed]
- Stoelting RK, Eger EI 2nd. Percutaneous loss of nitrous oxide, cyclopropane, ether and halothane in man. Anesthesiology 1969;30:278-83. [Crossref] [PubMed]
Cite this article as: Shi J, Alston TA. Antivitamin action of nitrous oxide in OMF surgery—a narrative review. J Oral Maxillofac Anesth 2022;1:34.


