
Recognition and management of the difficult airway—a narrative review and update on the latest guidelines
Introduction
The American Society of Anesthesiologists (ASA) defines the difficult airway as one in which a conventionally trained anesthesiologist will have difficulty with either face mask ventilation or tracheal intubation (1). Management of the difficult airway remains a key challenge for anesthesiologists. The incidence of difficult mask ventilation is reported to be 1.4–7.5% with 0.15% of patients being impossible to ventilate (2,3). The incidence of difficult intubation ranges from 1% to 8% and the incidence of failed intubation is 0.05–0.35% (4,5).
Evaluating and identifying a challenging airway is the essential first step to airway management. Although the difficult airway can be anticipated, many anesthesiologists still encounter unanticipated difficult airways. Furthermore, the management of the difficult airway has been ever evolving. Awake fiberoptic intubation (AFOI) or awake flexible bronchoscopic intubation, is one of the recommended strategies for securing an airway in patients suspected of being difficult to intubate, particularly if associated with difficult ventilation also. Fiberoptic intubation was popularized in the 1980’s (6).
Many guidelines from reputable societies have been issued over the last 30 years, and are presented in this review giving a board overview on the recognition and management of the difficult airway, as well as management of the unanticipated difficult airway. Addition of a brief overview of the new 2022 ASA difficult airway guidelines along with traditional difficult airway management techniques allows for a concise and thorough review of difficult airway management.
We hope to provide the reader with a concise overview of the recognition and management of the difficult airway reflecting on best practices and current recommendations for airway management. We present this article in accordance with the Narrative Review reporting checklist (available at https://joma.amegroups.com/article/view/10.21037/joma-23-3/rc).
Methods
The authors performed a focused literature review of guidelines for the management of the difficult airway from major related USA and international societies from January 1993–May 2022 as well as the most relevant references from the identified guidelines and to the topics discussed. We searched PubMed using keywords: awake fiberoptic intubation, flexible bronchoscopic intubation, flexible intubation scopes, emergency invasive airway, difficult airway management. The search was limited to the English language.
Articles included retrospective studies, prospective studies, clinical trials and case reports (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 01 May, 2022 |
| Databases searched | PubMed |
| Search terms used | Difficult airway, awake fiberoptic intubation, flexible bronchoscopic intubation, flexible intubation scopes, emergency invasive airway, emergency airway management |
| Timeframe | 1993–2022 |
| Inclusion and exclusion criteria | Inclusion: English language only, clinical trials, retrospective studies, prospective studies, case studies, all ages, sexes |
| Exclusion: does not meet inclusion standards | |
| Selection process | All authors |
Discussion
Despite considerable progress in difficult airway management, it is still a major contributor to patient morbidity and mortality, particularly brain damage and death (7). In fact, 25–46% of anesthetic related deaths are associated with difficult airways (8). Moreover, there has been an increase in the body of knowledge and advances in technology. Thus, major international anesthesiology societies have issued/updated their guidelines with the hope that the updated guidelines will keep practitioners abreast of the new concepts, technology and trends in safe airway management. The ASA first published practice guidelines for the management of the difficult airway was in 1993 (9). In those first guidelines, the fiber optic scope received wide commendation in its role as an essential tool for airway management. The guidelines were updated in 2003 (10), 2013 (1), and again in 2022 (11).
A note on the updated ASA guidelines
The ASA in line with their concern for and leadership in the patient safety movement in the USA and the world issued the updated 2022 ASA Difficult Airway Practice Guidelines.
As always, these guidelines were based on the available evidence, and when evidence is lacking, expert opinion including survey results from airway management experts from around the world and ASA members as well as those of the collaborating USA and international societies.
We herein highlight some of the important concepts included in this 2022 update.
Updates highlights:
- International 15 members task force;
- 12 national and international societies;
- More inclusive of clinicians, and settings;
- Decision tool;
- Emphasis on the number of attempts;
- Emphasis on the passage of time: earlier invasive airway;
- Infographics for both adult and pediatric patients;
- New pediatric algorithm;
- Emphasis on provision of O2 throughout the management of the difficult airway, including extubation;
- More robust recommendations for the extubation of the difficult airway;
- Human factors in difficult airway management;
- New list of suggested contents of standard anesthetizing location.
The guidelines as always start with highlighting the importance of airway evaluation, and if we may add, by the clinician responsible for managing the airway at hand. Moreover, detailed recommendations are provided for preparing for management of the difficult airway, as it is said, that failure to plan, is a plan to fail. The decision-making tool is a new feature that should be examined very carefully. It includes the consideration for awake airway management in patients with high risk for aspiration, and/or those who cannot tolerate a brief apneic episode.
The ASA 2022 new pediatric difficult airway algorithm and infographic is a welcomed addition to the very popular “ASA difficult airway algorithm”. It follows almost the same principles regarding managing adult patient in terms of airway evaluation, and management, albeit with a focus on the concept of functional vs. physiologic airway obstruction reminding clinicians of the need to monitor the patient’s depth of anesthesia and muscle relaxation during the process of managing the difficult airway.
Also, recommendations were provided for the extubation of the difficult airway highlighting that it is an elective procedure. And finally, follow up steps after a difficult airway encounter.
A list of human factors that may contribute to the management of the difficult airway is provided, as well as suggested contents for the standard anesthetizing location, and difficult airway cart.
Other national and international societies’ guidelines
The Canadian Airway Focus Group (CAFG) initially also made recommendations in 1998 with updates in 2013 (12,13). They were further updated in 2021 (14,15).
The Difficult Airway Society (DAS), based in the United Kingdom also issued guidelines for management of the difficult airway in 2004 that was updated in 2015 (16).
In 2022, Project for Universal Management of Airways (PUMA) worked with several international airway societies including DAS, Society for Airway Management, European Airway Management Society, All India Difficult Airway Association, CAFG, Safe Airway Society, International Airway Management Society to develop a consensus guideline for providing a foundation for airway management (17).
The three main guidelines from the ASA, CAFG, and DAS are independently developed by three totally different groups of national/international airway experts. They are all well thought out and dependent on the available literature evidence and when evidence was lacking, then consensus of expert opinion. Resulting guidelines differed as they depended on the standards and local practices in their respective country of origin. The DAS guidelines focused on the unanticipated difficult airway, while the ASA and CAFG guidelines addressed both the anticipated and unanticipated difficult airway. The ASA and CAFG included within them recommendations for the extubation of the difficult airway, while the DAS decided to have a separate guideline for the same as well as their own version of guidelines for the awake tracheal intubation.
One example of the differences in recommendations between different guidelines is the establishment of emergency invasive airway access. The DAS guidelines as well as the Canadian guidelines strongly recommend the scalpel bougie technique, while the ASA calls for other alternative techniques, this is discussed in detail elsewhere in this review.
Another example of differences in guidelines recommendations is regarding the patient with full stomach and at high risk for aspiration. Both the Canadian and the ASA guidelines recommend awake intubation to minimize the risk for aspiration, while the DAS guidelines recommends rapid sequence induction with cricoid pressure as means to prevent regurgitation and aspiration.
Moreover, the ASA describes the failed airway emergency as cannot intubate/cannot ventilate (CICV) while the CAFG and DAS Guidelines describes it as cannot intubate/cannot oxygenate (CICO). At the risk of being biased, as our senior author (B.A.) was a member of the ASA task force to update the difficult airway practice guidelines, the authors prefer CICV, as oxygenation (measured by O2 saturation pulse oximetry) can be misleading and it would remain stable for some time while there is no ventilation (18), and as soon as it starts to drop it does so rapidly and may not allow for adequate time for intervention with a deadly sequela. However, lack of ventilation would give an early warning of the challenge and thus allows for time to move on to the next step in the planned airway strategy and/or early decision to proceed with invasive airway. Additionally, ventilation includes oxygenation within it as ventilation without oxygenation is a lower airway/lung parenchyma challenge, not an upper airway challenge that these guidelines are concerned with. Regardless, it seems like most clinicians will follow the guidelines of the society that serve their practice/country.
Recognition of the difficult airway
According to the 2022 ASA Practice Guidelines for Management of Difficult Airways, the definition of difficult airways can be subdivided into several categories. Difficult facemask ventilation includes poor mask seal/excessive gas leak and/or excessive resistance to gas flow. Difficult laryngoscopy is the inability to properly visualize any vocal cord structures despite multiple efforts. Difficult supraglottic airway (SGA) placement involves an inability to correctly position the SGA, placement requiring multiple attempts, poor SGA seal/excessive gas leak, or excessive resistance to gas flow. Difficult tracheal intubation is the inability to correctly place an endotracheal tube (ETT) despite multiple attempts. There is no further definition of what defines multiple attempts in the ASA guidelines, however, during difficult airway management, the guideline recommends limiting attempts with any technique class (i.e., face mask, SGA, ETT) to three, with one additional attempt by a clinician with higher skills. Difficult tracheal extubation is the management of extubation of an intubated patient who is known or suspected to have any variety of the definitions of the difficult airway. Difficult invasive airway access involves difficulty or an inability to establish a surgical airway due to abnormal patient features such as morbid obesity, large goiters or other anterior neck masses, previous neck surgery and or radiation, etc. (11).
Proper prediction and identification of the difficult airway allows for appropriate planning. Thus, first attempt success and patient safety can be optimized, and risks of adverse outcomes can be minimized or avoided.
The ASA difficult airway guidelines highlight various demographic and clinical characteristics which may be evaluated to assess the likelihood of difficult airway. These include age, body mass index, weight, and height. Clinical characteristics assessed included a history of difficult intubation, distorted airway anatomy, snoring, obstructive sleep apnea, diabetes mellitus, or findings from diagnostic tests (e.g., radiography, computed tomography), patient interviews, and questionnaires. Measurement of facial and jaw features included mouth opening, the ability to prognath, head and neck mobility, prominent upper incisors, presence of a beard, and an upper lip bite test. Anatomical measures included Mallampati and modified Mallampati scores, thyromental distance, sterno-mental distance, inter-incisor distance, neck circumference, ratio of neck circumference to thyromental distance, ratio of height to thyromental distance, hyo-mental distance, and hyo-mental distance ratio. Measurements obtained from ultrasound include skin-to-hyoid distance, tongue volume, and distance from skin to epiglottis. Acquired and congenital conditions have also been identified by case reports as having an association with difficult airway (Table 2).
Table 2
| Patient history associated with a higher risk of difficult airways | Mechanisms of increased risk of difficult airways |
|---|---|
| History of a difficult airway | – |
| Diabetes | • Limited joint mobility due to glycosylation of collagen |
| • Delayed gastric emptying | |
| Rheumatoid arthritis | • Hypermobility or immobility of the joints of the jaw, larynx, and neck due to chronic autoimmune pathology |
| Ankylosing spondylitis | • Fusion and rigidity of spine, TMJ and cricoarytenoid joint due to spondyloarthropathy |
| Temporomandibular disorders | • Impaired mouth opening |
| Bleeding risk in the airway | • Visualization of vocal cords |
| • Aspiration risk | |
| Congenital abnormalities (Treacher Collins syndrome, Pierre Robin syndrome, Goldenhar syndrome, etc.) | • Craniofacial abnormalities |
| Masses of the head, neck, and airway (with and without radiation) | • Reduced neck range of motion |
| • Anatomical distortion | |
| Burns | • Inhalational injury |
| • Traumatized tissue | |
| Acromegaly | • Overgrowth of mucosa and the soft tissues of the pharynx, larynx, and vocal cords |
| Obesity | • Multifactorial anatomical and physiologic changes related to adipose tissue deposition |
| Obstructive sleep apnea | • Multifactorial |
| Pregnancy | • Upper airway edema |
| • Aspiration risk | |
| Aspiration risk | – |
| Psychosocial context | – |
Reference: Chapter 9 in Hagberg and Benumof’s Airway Management. 5th Edition. 2023 (19). TMJ, temporomandibular joint.
Imaging studies may also be useful. Observational studies have found utility in radiographic or computed tomography imaging to further reveal any anatomic variations which may lead to a difficult airway. However, difficulty may be underestimated in situations where the appearance of the lesion or anatomical variation in question varies with phases of the respiratory cycle for example. In particular, the pre-operative trans nasal endoscopic exam may be particularly helpful in identifying pathology which may lead to airway management difficulties (20,21). However, others have questioned the value of such evaluation (22).
Risk assessment for likelihood of a difficult airway must take into account many factors. While no single factor exists that best predicts a difficult airway, assessing a combination of multiple variables will best help the anesthesiologist to adequately prepare for airway management. Further studies would be useful to most accurately and concisely assess a patient’s risk of being a difficult airway (23).
Strategies for difficult airway management
Strategies and techniques for difficult airway management should be based on the anesthesiologist’s experience, available resources and equipment and competency of help. If a difficult airway is encountered unexpectedly, the ASA Practice Guidelines for Difficult Airway Management can be instrumental in formulating the strategies. Pre-assessment and preparation is of key importance: ensuring equipment is available in the room, patient is properly positioned, and supplemental oxygen is administered throughout the entire process of airway management (11).
Noninvasive devices
Noninvasive devices for airway management of patients with anticipated or unanticipated and emergency difficult airways include rigid laryngoscope blades of alternative design and size, adjuncts (e.g., introducers, bougies, stylets, and different tracheal tubes), video laryngoscopes, flexible intubation scopes, SGA, lighted or optical stylets, rigid bronchoscopes, and combination techniques (11) (Figures 1-4).
Invasive interventions
Invasive airway management interventions for anticipated difficult airway management include retrograde wire–guided intubation, awake cricothyrotomy/tracheostomy, and/or jet ventilation (11) (Figures 5,6).
Anticipated difficult airway management
The difficult airway management plan should take into consideration the type of surgery and the patient’s condition, patient cooperation/consent, patient age and the anesthesiologist’s skills.
Awake intubation should be considered when the patient is suspected to be difficult intubation and one or more of the following apply: difficult ventilation with face mask or SGA, increased risk of aspiration, unlikelihood of patient being able to tolerate brief apnea, or expected difficulty with emergency airway rescue (11).
Throughout all attempts of difficult airway management, one must always be cognizant of the passage of time, number of attempts and the oxygen saturation.
AFOI oral and nasal intubation
All anesthesiologists should master the skill of performing AFOI. A recent review by Cabrini et al. indicated that AFOI has been shown to have an overall failure rate of 12 of 2045 intubations (0.59%) and a severe adverse event rate of 7 of 2,045 (0.34%). No permanent consequences or death was recorded in this review (24).
Mastery of AFOI takes skill and practice with expert knowledge of the nervous innervation and anatomy of the airway as well as excellent technical skills with the fiber optic bronchoscope. It should be noted that intubating bronchoscopes no longer employ the fiber-optic technology but rather a video chip, thus a new terminology proposed is the “flexible intubating scope”.
Innervation of the airway
The most important first step in conducting an AFOI, is the ability to make the decision that an AFOI is indicated. Next, knowledge of innervation of the airway is essential to successful performance of an AFOI.
The innervation of the nasal cavity originates from the trigeminal nerve. The oral cavity is innervated by the glossopharyngeal nerve which provides sensory innervation to the posterior third of the tongue, vallecula, anterior surface of the epiglottis, posterior and lateral walls of the pharynx, and the tonsillar pillars.
The larynx is innervated by the vagus nerve. Its superior laryngeal branch further branches into the internal (sensory) and external (motor) branches. The internal provides the sensory innervation to the base of the tongue, vallecula, epiglottis, aryepiglottic folds, arytenoids, and down to, but not including the vocal cord. The external branch provides motor innervation to the cricothyroid (CT) muscle.
Another branch of the vagus nerve, the recurrent laryngeal nerve, innervates the trachea and the vocal cords. The recurrent laryngeal nerve provides motor innervation to all muscles of the larynx except the CT muscle and sensory innervation to the vocal cords and the trachea.
Preparation for awake intubation
Equipment (Table 3)
Table 3
| Flexible intubating bronchoscope |
| Suction tubing to be attached to suction port of scope |
| Antifogging agents |
| Lubricating agents |
| Oral airways (Williams, Berman, Ovasapian) |
| Nasal airways (if nasal intubation is to be performed) |
| Supplemental oxygen (nasal cannula, face mask or high flow nasal cannulae) |
| Endotracheal tube (Parker or standard) |
| Topical anesthetic administering devices (atomizing devices, nebulizer, mucosal atomization device) |
| Adjuncts such as cotton pledgets and cotton tipped swabs |
| Sedation medications (provider specific; goal of maintaining spontaneous ventilation) |
| Topical and/or airway nerve block anesthetic agents |
The key to a successful AFOI is preparation. Most institutions will have an established airway “cart” or “trolley” housing commonly used equipment (25) (Figure 7). The most crucial piece of equipment is the flexible intubating bronchoscope. The bronchoscope allows visualization of the entire airway above and below the vocal cords. It also acts as a conduit guiding the ETT into the airway. Additionally, the scopes house channels that allow suctioning or administering of local anesthesia.
In our routine practice, we prefer the largest size bronchoscope feasible to take advantage of superior visualization and stronger suction. A significantly narrowed airway warrants a smaller bronchoscope, at the expense of less optimized visualization and suction. Whenever possible, we recommend choosing the ETT most closely matching the bronchoscope, as the bigger the gap between the ETT and the bronchoscope, the more chance of the tube becoming “hung-up” at arytenoids during insertion.
Antifogging agents are helpful as they serve to minimize condensation and fogging of the scope lens (Figure 8). Lubricating agents allow for easier passage of the scope and tube. A variety of oral airways, bite blocks and tooth guards serve to both protect the scope, provide a midline path for the scope and provide relief from obstruction. Nasal airways may be used to aid in lubricating and anesthetizing the nares prior to a nasal intubation.
Compared to the last edition, the new 2022 ASA practice guideline emphasized supplemental oxygen administration before initiating and throughout difficult airway management, including the extubation process. Therefore, oxygen delivery devices such as nasal cannulae, specialized facemasks or high flow nasal oxygen (HFNO) should be readily available. The 2020 Difficulty Airway Society guidelines for awake tracheal intubation in adults recommended applying HFNO early and titrating the HFNO from 30–70 L/min (26).
Although standard ETT may be used for AFOI, specialized tubes such as the Parker tube can facilitate passage of the tube into the trachea. The bull nose shape of the Parker tube prevents the tube from getting lodged on the arytenoids or epiglottis (27).
A variety of equipment exists for topicalization of the airway such as atomizing devices, nebulizers, mucosal atomization devices and nasal aerosolization devices (Figure 9). Adjuncts to topicalization include cotton pledgets and cotton tipped swabs.
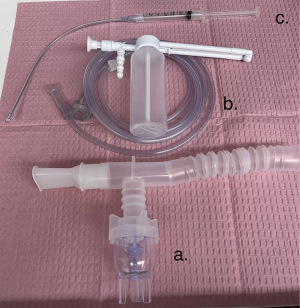
Sedation for awake intubation
Appropriate airway anesthesia and sedation decrease the patients’ anxiety and discomfort during an AFOI. The goal of sedation is to have a patient who is comfortable and cooperative and who can also maintain their own airway. Sedation must be titrated carefully to maintain spontaneous ventilation.
The drugs used to sedate are provider specific. No one drug has been shown to be superior to another. Judicious titration with meticulous monitoring for side effect is essential with the goal of maintaining a comfortable and cooperative patient. Benzodiazepines and opioids are commonly used. These drugs have reversal agents should over sedation occur. Opioids have the added bonus of being antitussive. Frequently short acting agents such as midazolam and fentanyl are chosen. Infusions of carefully titrated propofol can be used for sedation, however, one has to consider the narrow therapeutic index for propofol when used for sedation. Infusions drugs such as remifentanil or dexmedetomidine offer very fine levels of titration but do require more time to reach their steady state. Dexmedetomidine has the added advantages of sedating a patient without causing respiratory depression (28), as well as reducing secretions. Compared to remifentanil, dexmedetomidine may be more effective in reducing the incidence of hypoxemia and memory recall of endoscopy. Its use in AFOI has been shown to provide better intubating conditions and hemodynamic stability compared to fentanyl with ketamine (29-31).
Remifentanil is a potent short acting narcotic, which is easily titrated. It suppresses airway reflexes as well and produces analgesia; however, it has minimal effect on memory and cognitive function. Remifentanil may take a longer time to achieve adequate sedation level to allow for a comfortable and safe awake intubation, a characteristic it shares with dexmedetomidine. Moreover, it may cause respiratory depression and chest wall rigidity (32,33). Target controlled remifentanil infusions can also be used to perform sedation for AFOI (34).
The Bispectral Index (BIS) monitor (Medtronic, Boulder, CO, USA) serves as an effective objective tool for titrating the depth of conscious sedation. A BIS range of 80–86 is appropriate for performing the AFOI (35).
Supplemental oxygen during AFOI is recommended especially if sedation is to be used. Nasal cannula, facemask or HFNO have been used. HFNO is particularly useful for the patient who has poor pulmonary reserve or hypoxemia at baseline (26,36-38). The endoscopic mask has been successfully and safely used in AFOI, with advantages of stable blood pressure and potential prevention of desaturation (39). Oxygen insufflation via the working channel of a fiber optic scope is another useful method to maintain oxygenation that has been described (40).
Upper airway anesthesia for AFOI
One can anesthetize the upper airway by either performing airway nerve blocks or using topical anesthesia. Clinicians often worry that convincing a patient for the need for AFOI is difficult enough without the need to convince the patient they will have to tolerate a number of injections as well.
A number of blocks have been described. The glossopharyngeal nerve is anesthetized by injecting local anesthetic at the base of the tonsillar pillars. The superior laryngeal nerve block is performed by placing local anesthetic beneath the greater cornu of the hyoid bone bilaterally. The recurrent laryngeal nerve is anesthetized via a trans-tracheal block where local anesthetic is placed on the trachea after piercing the CT membrane.
In the authors’ clinical experience, we have found that atomization is a very efficient and efficacious way of preparing a patient for awake intubation and it avoids the fear and discomfort caused by nerve blocks.
Some clinicians advocate administering an antisialagogue to help dry secretions prior to beginning topicalization. Glycopyrrolate 0.2 mg intravenous (IV) is used if there are no contraindications. If nasal intubation is planned, a vasoconstrictive agent should be placed in the nares. Vasoconstrictors used for nasal intubation include cocaine, oxymetazoline 0.05%, xylometazoline 0.1% and phenylephrine 0.5%. Cocaine is useful in that it has both vasoconstrictor and local anesthetic properties, thus favorable for use with nasal intubation. It is available as a 4% solution. Its maximum recommended dose for nasal application is 1.5 mg/kg.
Benzocaine is available as a 10%, 15% or 20% spray. It has a rapid onset of action but is limited by the risk for the development of methemoglobinemia and its short duration of action (5 minutes). Maximum dose should not exceed 200 mg per dose (41). Tetracaine comes in concentrations of 0.5%, 1% and 2%. Doses should be limited to 20–40 mg per dose. Cetacaine is a combination of benzocaine, butamben and tetracaine. Each one-second spray contains an average of 200 mg of product. Patient dosing should not exceed 400 mg.
Lidocaine is perhaps the most commonly used local anesthetic used for topicalization of the airway. It has a rapid onset of action and a wide therapeutic index. Its duration of action is up to 1 hour with a peak at 15 minutes. It comes as a gel or liquid, both of which may be used on the airway, in concentrations of 1%, 2% and 4% (Figure 10). The maximum dose allowable for topicalization is less well established. The British Thoracic Society recommends 8.2 mg/kg. Others recommend a lower limit of 4–5 mg/kg (42-44).
The nasal cavity can be anesthetized by using long cotton tipped applicators soaked in either 4% cocaine or 4% lidocaine with 1:200,000 epinephrine. They are then inserted superiorly and posteriorly in the nasopharynx and left for 5 minutes to numb the nasal cavity. Lidocaine can also be administered in a nebulizer or atomizer to further topicalize the airway. It is important to start this early as it may take some time to anesthetize the airways. Further, touch up topicalization can be performed with an atomizer filled with 4% lidocaine. Adequacy of anesthesia is determined by testing pharyngeal sensation with a tongue blade. Tolerance of an oral airway demonstrates adequate anesthesia of the hypopharynx. A malleable atomization device can then be inserted through the oral airway to deliver additional lidocaine to the larynx (Table 4).
Table 4
| Non-invasive technique | Invasive technique |
|---|---|
| Nebulizer | Superior laryngeal nerve block |
| Atomizer | Glossopharyngeal nerve block |
| Spray-as-you-go | Transtracheal block |
Reference: Chapter 24 in Hagberg and Benumof’s Airway Management. 5th Edition. 2023 (25).
Despite these techniques, it is difficult to anesthetize the larynx due to obstruction by the tongue and other soft tissues. As the bronchoscope is inserted, further lidocaine can be sprayed on the vocal cords through the bronchoscope once they are visualized.
Technique for oral and nasal AFOI
Preparation
Once adequate topicalization and sedation have been achieved, it is time to proceed with the AFOI. The bronchoscope should be defogged and lubricated, the ETT lubricated, and cuff checked. Suction should be connected to the bronchoscope. The patient should be positioned such that they are comfortable, and the anesthesiologist has adequate access. Often a slight sitting up position works best. The head at extension position has been found to have the best view of glottic opening compared to the head in a neutral position or sniffing position during AFOI (45).
Awake oral intubation
The bronchoscope is held in the provider’s left hand with the thumb used to depress or lift the lever thereby moving the tip of the bronchoscope. The distal end of the bronchoscope is held with the provider’s right hand. Keeping the insertion cord as straight as possible. The bronchoscope is inserted into the oral airway that has been placed in advance. The bronchoscope should be advanced through the oral cavity and through the vocal cords into the trachea. A jaw thrust may help achieve a better view.
Once the bronchoscope has been placed above the carina, the pre-loaded ETT is railroaded over the scope into the patient’s trachea. Sometimes gentle twisting or corkscrew motion of the tube will facilitate its advancement through the vocal cords. The location of the ETT is verified first by the flexible intubating scope and second by capnography. General anesthesia is then induced.
Awake nasal intubation
Nasal intubation may be required due to surgical or patient factors. Patient issues such as severe trismus may make oral intubation impossible. The surgeon may request nasal intubation to facilitate the surgical technique. Difficulties with nasal intubation include patient discomfort during intubation, epistaxis and limitation in ETT size that can be used. Choosing the correct size tube may be difficult, the tube should be long enough to reach past the vocal cords, but not too large in diameter that may injure nasal structures.
The larger nare is identified on the patient and its patency is confirmed by the patient determining that their ability to breathe through it is equal or better to the other nare. After adequate vasoconstriction and topicalization, the nare is gently tested by passing a lubricated nasal trumpet. The ETT is then placed in the nares and advanced to the posterior pharynx. The bronchoscope is then placed through the ETT and advanced through the glottic opening. The tube is then advanced over the scope into the trachea. Any bleeding that occurs will make airway visualization more challenging. Alternatively, the tube can be loaded onto the bronchoscope; the bronchoscope itself is first passed through the nose and into the glottic opening and then the tube is advanced over it through the nose and into the trachea. This technique may result in less bleeding.
Unanticipated and emergency difficult airway management
The ASA guidelines recommend airway management of an unanticipated or emergency difficult airway consists of the following interventions:
- Calling for help—an additional set of hands will be beneficial and make dealing with the airway easier.
- Optimization of oxygenation—ensure that the patient’s oxygenation is adequate. Consider interventions like HFNO which may improve the current state.
- Use of a cognitive aid—cognitive aids are prompts designed to help users complete a task or series of tasks, especially during a stressful situation (46).
- Noninvasive airway management devices—optimize mask fit, oral airway, nasal airway, SGA, KingTube, etc.
- Combination techniques—fiberoptic bronchoscopy through SGA, fiberoptic bronchoscopy in concert with video laryngoscopy, etc.
- Invasive airway management interventions—front of neck access, trans tracheal jet ventilation, etc. (11).
CICV
The CICV situation is a rare but life-threatening event estimated to occur, on average, once in an anesthesiologist’s career. This prevalence is likely to be increased in high-risk areas such as head and neck cancer, trauma, and critical care. Cricothyroidotomy is the last resort in most cases upon CICO declaration. The cricothyrotomy techniques can be divided into needle-based and scalpel-based.
The needle-based technique can be divided into the narrow-bore cannula method and the wide-bore cannula method depending on the size of the cannula inserted through the CT membrane. Narrow-bore (<4 mm) cannula method, using a 14 g angiocath is one option, however, this has a well-described failure rate. Due to the small size of the cannula, this method mandates a high-pressure ventilation modality, e.g., jet ventilation, which is associated with a higher complication rate, especially barotrauma and subcutaneous emphysema (47,48).
Wide-bore cannula over a guidewire, using Seldinger technique, is more intuitive than the scalpel-based technique to anesthesiologists. However, the Seldinger technique requires fine motor control, making it more challenging in an extremely high-stake stressful situation. Compared to the narrow-bore cannula, it has the advantage of not needing jet ventilation and the complication rate is lower (49,50).
The scalpel-based method involves incising the CT membrane using a scalpel to create an opening into the airway below the vocal cords. The most critical point is to identify the CT membrane. If the CT membrane is easy to palpate or identifiable via ultrasonography (USG), the DAS recommends making a transverse incision through the skin and CT membrane. If the CT membrane is impalpable or if other techniques have failed, DAS recommends making a caudad to cephalad vertical midline 8–10 cm skin incision and then using blunt dissection with fingers of both hands to separate tissues and identify the CT membrane, followed by transverse incision of the CT membrane (50). A bougie is then inserted into the trachea followed by a size 6.5 ETT.
In the 2015 newest DAS guidelines, the recommendation of needle/cannula-based cricothyroidotomy has been completely removed from the previous edition published in 2004. The 2015 guidelines specifically recommended scalpel cricothyroidotomy technique mostly due to the findings from NAP4 study, which suggested that the scalpel method was more successful than the needle-based one. However, the caveat is that all these scalpel-based procedures were performed by surgeons. Therefore, it is currently unclear if the improved procedural success is related to the procedure or the operator (16,48,50,51).
Extubation of the difficult airway
The management of the difficult airway is not complete with the placement of the ETT. Extubation must also be considered. “At-risk” extubation is a scenario where the ability of a patient to maintain their airway and/or oxygenation after tracheal extubation is uncertain. This term applies to any potentially difficult reintubation as well as any risk factor such as acid-base derangement, aspiration risk, unstable hemodynamics, or temperature control (52).
The risk is highest if both factors are present. The key decision to be made is whether it is safer to extubate, or preferable for the patient to remain intubated, or an elective tracheostomy performed. According to the DAS guidelines, if it is considered safe to extubate, then awake extubation or one of the advanced techniques can be utilized. None of these recommended techniques is without risk. If it is considered unsafe to extubate, the options are to postpone extubation or perform a tracheostomy (52).
Extubation failure is defined as the inability to tolerate removal of the ETT and requires immediate tracheal reintubation. The mechanisms of this failure include all causes of airway obstruction, such as laryngospasm, airway edema, bleeding leading to hematoma or clots, accumulated respiratory secretions, tracheal collapse due to tracheomalacia, and airway soft tissue collapse secondary to the effects of anesthetics, opioids, and muscle relaxants (53).
As all difficult intubation patients are “At-risk” extubation, our goal when extubating is to avoid extubation failures. A number of factors have been identified by various airway guidelines that may contribute to the success or failure of an extubation (11).
- Assessment of patient readiness for extubation.
- The presence of a skilled individual to assist with extubation.
- Selection of an appropriate time and location for extubation.
- Planning for possible reintubation.
- Elective tracheostomy.
- Awake extubation.
- Supplemental oxygen throughout the extubation process.
- Extubation with an airway exchange catheter (AEC).
AECs are the main devices described in the literature that allow for rapid reintubation in the setting of a failed extubation. Continuous access to the airway postextubation via an AEC is recommended for selected high-risk patients by various airway guidelines. This strategy serves as a bridge for potential reintubation. A review of AEC use, suggested that the indwelling AEC appears to increase the first-pass success rate in patients with known or suspected difficult airway and decrease the incidence of complications in patients who failed extubation and require reintubation (1,13,52,54-56).
SGA exchange, also called Bailey’s maneuver, is an alternative method to extubation. It involves the replacement of an ETT with a SGA to maintain a patent, less invasive airway, and better patient tolerance with a preferable hemodynamic profile (57-59).
A cuff leak test may be performed prior to extubation. The principle of the cuff leak test is that the leak detected around the ETT with a deflated cuff is dependent on the area of the patent trachea and thus is inversely related to the degree of airway obstruction due to laryngeal edema. It is used for patients who are at risk for airway edema (60).
Utility of airway USG
USG has already become a daily practice for anesthesiologists and intensivists but is still under-utilized for airway management and assessment. Utilization of point of care ultrasound (POCUS) in the difficult airways may include:
- Rapid screening for difficult laryngoscopy.
- Identify CT membrane to facilitate urgent cricothyrotomies.
- Confirm the position of the ETT.
- Evaluate aspiration risk.
Current studies have shown that POCUS can provide reliable information about the volume and nature of gastric contents. With this technology, anesthesiologists can make individual decisions to minimize the risk of perioperative aspiration (61-68).
Strengths and limitations
Our review covers a broad range of airway related literature over a period of 20+ years as well as focuses on the most recent ASA guidelines. This wide span of coverage allows the reader a comprehensive overview of airway management techniques. Limitations include the fact that many articles were not included, thus we may have a limited overview of how the difficult airway is managed. Articles in languages other than English were excluded and thus cultural differences in airway management me be amiss. Again, presented here are only recommendations and guidelines based on the literature and the reader must establish best practices for their own institutions.
Conclusions
We present a thorough review of evaluation and management of the difficult airway in this review article including the topic of difficult extubation. Careful assessment, decision making, anticipation and planning, as well as the skills of the anesthesiologists considering the human factors in airway management are the keys to favorable outcomes. Safe management of the difficult airway can be accomplished by having a thorough pre-operative evaluation with advance planning. Providing oxygenation throughout the airway management process, being cognizant of time and number of attempts are extremely important principles. Early consideration for surgical airway should be made in a “cannot intubate, cannot ventilate” clinical situation when encountered as a life-saving intervention.
This review gives a thorough overview of difficult airway management, including presentation on differing guidelines as well as specific focus on the ASA difficult airway 2022 updated guidelines. This will allow the reader to best decide what equipment, training and policies they wish to establish for their home institutions.
Acknowledgments
The authors thank Katherine Higgins and Erika Cox for their support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://joma.amegroups.com/article/view/10.21037/joma-23-3/rc
Peer Review File: Available at https://joma.amegroups.com/article/view/10.21037/joma-23-3/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://joma.amegroups.com/article/view/10.21037/joma-23-3/coif). U.G. received consulting fees from Baxter Health IV bag advisory board. B.A. received Royalties or licenses from Cambridge University Press and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Medtronic. He is also the Board of Director Member of American Society of Anesthesiologists (ASA), Society for Airway Management (SAM) and Society for Ambulatory Anesthesia (SAMBA). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251-70. [Crossref] [PubMed]
- Shah PN, Sundaram V. Incidence and predictors of difficult mask ventilation and intubation. J Anaesthesiol Clin Pharmacol 2012;28:451-5. [Crossref] [PubMed]
- Kheterpal S, Martin L, Shanks AM, et al. Prediction and outcomes of impossible mask ventilation: a review of 50,000 anesthetics. Anesthesiology 2009;110:891-7. [Crossref] [PubMed]
- Seo SH, Lee JG, Yu SB, et al. Predictors of difficult intubation defined by the intubation difficulty scale (IDS): predictive value of 7 airway assessment factors. Korean J Anesthesiol 2012;63:491-7. [Crossref] [PubMed]
- Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: a closed claims analysis. Anesthesiology 2005;103:33-9. [Crossref] [PubMed]
- Calder I. Murphy P. A fibre-optic endoscope used for nasal intubation. Anaesthesia 1967; 22: 489-91. Anaesthesia 2010;65:1133-6. [Crossref] [PubMed]
- Joffe AM, Aziz MF, Posner KL, et al. Management of Difficult Tracheal Intubation: A Closed Claims Analysis. Anesthesiology 2019;131:818-29. [Crossref] [PubMed]
- Cook TM, MacDougall-Davis SR. Complications and failure of airway management. Br J Anaesth 2012;109:i68-85. [Crossref] [PubMed]
- Practice guidelines for management of the difficult airway. A report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 1993;78:597-602.
- Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003;98:1269-77. [Crossref] [PubMed]
- Apfelbaum JL, Hagberg CA, Connis RT, et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022;136:31-81. [Crossref] [PubMed]
- Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth 1998;45:757-76. [Crossref] [PubMed]
- Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management--part 1--difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anaesth 2013;60:1089-118. [Crossref] [PubMed]
- Law JA, Duggan LV, Asselin M, et al. Canadian Airway Focus Group updated consensus-based recommendations for management of the difficult airway: part 1. Difficult airway management encountered in an unconscious patient. Can J Anaesth 2021;68:1373-404. [Crossref] [PubMed]
- Law JA, Duggan LV, Asselin M, et al. Canadian Airway Focus Group updated consensus-based recommendations for management of the difficult airway: part 2. Planning and implementing safe management of the patient with an anticipated difficult airway. Can J Anaesth 2021;68:1405-36. [Crossref] [PubMed]
- Henderson JJ, Popat MT, Latto IP, et al. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004;59:675-94.
- Chrimes N, Higgs A, Law JA, et al. Project for Universal Management of Airways - part 1: concept and methods. Anaesthesia 2020;75:1671-82. [Crossref] [PubMed]
- Abdelmalak B, Wang J, Mehta A. Capnography monitoring in procedural sedation for bronchoscopy. J Bronchology Interv Pulmonol 2014;21:188-91. [Crossref] [PubMed]
- Hagberg CA. Airway Assessment and Prediction of the Difficult Airway. In: Hagberg CA, editor. Hagberg and Benumof's Airway Management. 5th Edition. New York: Elsevier; 2023.
- Gemma M, Buratti L, Di Santo D, et al. Pre-operative transnasal endoscopy as a predictor of difficult airway: A prospective cohort study. Eur J Anaesthesiol 2020;37:98-104. [Crossref] [PubMed]
- Rosenblatt W, Ianus AI, Sukhupragarn W, et al. Preoperative endoscopic airway examination (PEAE) provides superior airway information and may reduce the use of unnecessary awake intubation. Anesth Analg 2011;112:602-7. [Crossref] [PubMed]
- Abdelmalak B. The case against preoperative endoscopic airway examination (PEAE) in the “ENT airway”. JOHNA 2019;3:e22.
- Heinrich S, Birkholz T, Irouschek A, et al. Incidences and predictors of difficult laryngoscopy in adult patients undergoing general anesthesia: a single-center analysis of 102,305 cases. J Anesth 2013;27:815-21. [Crossref] [PubMed]
- Cabrini L, Baiardo Redaelli M, Ball L, et al. Awake Fiberoptic Intubation Protocols in the Operating Room for Anticipated Difficult Airway: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Anesth Analg 2019;128:971-80. [Crossref] [PubMed]
- Hagberg CA. Flexible Scope Intubation Techniques. In: Hagberg CA, editor. Hagberg and Benumof's Airway Management. 5th Edition. New York: Elsevier; 2023.
- Ahmad I, El-Boghdadly K, Bhagrath R, et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia 2020;75:509-28. [Crossref] [PubMed]
- Kristensen MS. The Parker Flex-Tip tube versus a standard tube for fiberoptic orotracheal intubation: a randomized double-blind study. Anesthesiology 2003;98:354-8. [Crossref] [PubMed]
- Abdelmalak B, Makary L, Hoban J, et al. Dexmedetomidine as sole sedative for awake intubation in management of the critical airway. J Clin Anesth 2007;19:370-3. [Crossref] [PubMed]
- Verma AK, Verma S, Barik AK, et al. Intubating conditions and hemodynamic changes during awake fiberoptic intubation using fentanyl with ketamine versus dexmedetomidine for anticipated difficult airway: a randomized clinical trial. Braz J Anesthesiol 2021;71:259-64. [Crossref] [PubMed]
- He XY, Cao JP, He Q, et al. Dexmedetomidine for the management of awake fibreoptic intubation. Cochrane Database Syst Rev 2014;2014:CD009798. [Crossref] [PubMed]
- Ebert T, Maze M. Dexmedetomidine: another arrow for the clinician's quiver. Anesthesiology 2004;101:568-70. [Crossref] [PubMed]
- Tang ZH, Chen Q, Wang X, et al. A systematic review and meta-analysis of the safety and efficacy of remifentanil and dexmedetomidine for awake fiberoptic endoscope intubation. Medicine (Baltimore) 2021;100:e25324. [Crossref] [PubMed]
- Johnston KD, Rai MR. Conscious sedation for awake fibreoptic intubation: a review of the literature. Can J Anaesth 2013;60:584-99. [Crossref] [PubMed]
- Shah SV, Lacey O. A decade of using a remifentanil target-controlled infusion technique for awake fibreoptic intubations. Anaesthesia 2021;76:284-5. [Crossref] [PubMed]
- Gnaneswaran HH, Jain G, Agarwal A, et al. Optimal level of bispectral index for conscious sedation in awake fiberoptic nasotracheal intubation. J Oral Biol Craniofac Res 2020;10:299-303. [Crossref] [PubMed]
- Badiger S, John M, Fearnley RA, et al. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth 2015;115:629-32. [Crossref] [PubMed]
- Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015;70:323-9. [Crossref] [PubMed]
- Russotto V, Cortegiani A, Raineri SM, et al. Respiratory support techniques to avoid desaturation in critically ill patients requiring endotracheal intubation: A systematic review and meta-analysis. J Crit Care 2017;41:98-106. [Crossref] [PubMed]
- Zou T, Huang Z, Hu X, et al. Clinical application of a novel endoscopic mask: a randomized controlled, multi-center trial in patients undergoing awake fiberoptic bronchoscopic intubation. BMC Anesthesiol 2017;17:79. [Crossref] [PubMed]
- Lee D, Baik J, Yun G, et al. Oxygen insufflation via working channel in a fiberscope is a useful method: A case report and review of literature. World J Clin Cases 2018;6:1189-93. [Crossref] [PubMed]
- Vallurupalli S, Manchanda S. Risk of acquired methemoglobinemia with different topical anesthetics during endoscopic procedures. Local Reg Anesth 2011;4:25-8. [Crossref] [PubMed]
- Doyle DJ. Airway anesthesia: theory and practice. Anesthesiol Clin 2015;33:291-304. [Crossref] [PubMed]
- Pani N, Kumar Rath S. Regional & topical anaesthesia of upper airways. Indian J Anaesth 2009;53:641-8.
- British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56:i1-21. [Crossref] [PubMed]
- Liu Z, Zhao L, Ma Z, et al. Effects of head positions on awake fiberoptic bronchoscope oral intubation: a randomized controlled trial. BMC Anesthesiol 2021;21:176. [Crossref] [PubMed]
- Marshall S. The use of cognitive aids during emergencies in anesthesia: a review of the literature. Anesth Analg 2013;117:1162-71. [Crossref] [PubMed]
- Ross-Anderson DJ, Ferguson C, Patel A. Transtracheal jet ventilation in 50 patients with severe airway compromise and stridor. Br J Anaesth 2011;106:140-4. [Crossref] [PubMed]
- Cook TM, Woodall N, Frerk C, et al. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth 2011;106:617-31. [Crossref] [PubMed]
- Melker JS, Gabrielli A. Melker cricothyrotomy kit: an alternative to the surgical technique. Ann Otol Rhinol Laryngol 2005;114:525-8. [Crossref] [PubMed]
- Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 2015;115:827-48. [Crossref] [PubMed]
- Groom P, Schofield L, Hettiarachchi N, et al. Performance of emergency surgical front of neck airway access by head and neck surgeons, general surgeons, or anaesthetists: an in situ simulation study. Br J Anaesth 2019;123:696-703. [Crossref] [PubMed]
- Difficult Airway Society Extubation Guidelines Group. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012;67:318-40. [Crossref] [PubMed]
- Cavallone LF, Vannucci A. Review article: Extubation of the difficult airway and extubation failure. Anesth Analg 2013;116:368-83. [Crossref] [PubMed]
- Langeron O, Bourgain JL, Francon D, et al. Difficult intubation and extubation in adult anaesthesia. Anaesth Crit Care Pain Med 2018;37:639-51. [Crossref] [PubMed]
- Kundra P, Garg R, Patwa A, et al. All India Difficult Airway Association 2016 guidelines for the management of anticipated difficult extubation. Indian J Anaesth 2016;60:915-21. [Crossref] [PubMed]
- Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg 2007;105:1357-62. table of contents. [Crossref] [PubMed]
- Kalra N, Gupta A, Sood R, et al. Comparison of Proseal Laryngeal Mask Airway with the I-Gel Supraglottic Airway During the Bailey Manoeuvre in Adult Patients Undergoing Elective Surgery. Turk J Anaesthesiol Reanim 2021;49:107-13. [Crossref] [PubMed]
- Modir H, Moshiri E, Yazdi B, et al. Comparing the efficacy and safety of laryngeal mask airway, streamlined liner of the pharyngeal airway and I-gel following tracheal extubation. Med Gas Res 2017;7:241-6. [Crossref] [PubMed]
- Bhat CB, Honnannavar KA, Patil MBP, et al. Comparison of the Laryngeal Mask Airways: Laryngeal Mask Airway-classic and Laryngeal Mask Airway-proseal in Children. Anesth Essays Res 2018;12:119-23. [Crossref] [PubMed]
- Schnell D, Planquette B, Berger A, et al. Cuff Leak Test for the Diagnosis of Post-Extubation Stridor: A Multicenter Evaluation Study. J Intensive Care Med 2019;34:391-6. [Crossref] [PubMed]
- Ezri T, Gewürtz G, Sessler DI, et al. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior neck soft tissue. Anaesthesia 2003;58:1111-4. [Crossref] [PubMed]
- Wu J, Dong J, Ding Y, et al. Role of anterior neck soft tissue quantifications by ultrasound in predicting difficult laryngoscopy. Med Sci Monit 2014;20:2343-50. [Crossref] [PubMed]
- Rai Y, You-Ten E, Zasso F, et al. The role of ultrasound in front-of-neck access for cricothyroid membrane identification: A systematic review. J Crit Care 2020;60:161-8. [Crossref] [PubMed]
- Campbell M, Shanahan H, Ash S, et al. The accuracy of locating the cricothyroid membrane by palpation - an intergender study. BMC Anesthesiol 2014;14:108. [Crossref] [PubMed]
- Hiller KN, Karni RJ, Cai C, et al. Comparing success rates of anesthesia providers versus trauma surgeons in their use of palpation to identify the cricothyroid membrane in female subjects: a prospective observational study. Can J Anaesth 2016;63:807-17. [Crossref] [PubMed]
- Sahu AK, Bhoi S, Aggarwal P, et al. Endotracheal Tube Placement Confirmation by Ultrasonography: A Systematic Review and Meta-Analysis of more than 2500 Patients. J Emerg Med 2020;59:254-64. [Crossref] [PubMed]
- Alerhand S. Ultrasound for identifying the cricothyroid membrane prior to the anticipated difficult airway. Am J Emerg Med 2018;36:2078-84. [Crossref] [PubMed]
- Zhang G, Huang X, Shui Y, et al. Ultrasound to guide the individual medical decision by evaluating the gastric contents and risk of aspiration: A literature review. Asian J Surg 2020;43:1142-8. [Crossref] [PubMed]
Cite this article as: Galway U, Wang M, Deeby M, Zura A, Riter Q, Abdelmalak B. Recognition and management of the difficult airway—a narrative review and update on the latest guidelines. J Oral Maxillofac Anesth 2023;2:29.











